Table 1.
Sequential Preparation of Alkynes 2{1–15} and 3{1–11} from Aryl Halides 1
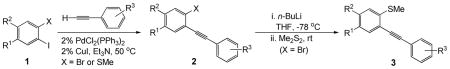 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | X | alkyne 2 | yield (%)a | alkyne 3 | yield (%)a |
| 1 | H | H | H | SMe | 2{1} | 88 | ||
| 2 | H | H | 4-MeO | SMe | 2{2} | 88 | ||
| 3 | H | H | 3-MeO | SMe | 2{3} | 77 | ||
| 4 | H | H | 2-MeO | SMe | 2{4} | 79 | ||
| 5 | MeO | H | 4-MeO | Br | 2{5} | 94 | 3{1} | 86 |
| 6 | MeO | H | 3-MeO | Br | 2{6} | 91 | 3{2} | 93 |
| 7 | MeO | H | 2-MeO | Br | 2{7} | 87 | 3{3} | 89 |
| 8 | MeO | H | 3,5-(MeO)2 | Br | 2{8} | 83 | 3{4} | 83 |
| 9 | H | MeO | 4-MeO | Br | 2{9} | 73 | 3{5} | 81 |
| 10 | H | MeO | 2-MeO | Br | 2{10} | 77 | 3{6} | 90 |
| 11 | MeO | MeO | 4-MeO | Br | 2{11} | 92 | 3{7} | 87 |
| 12 | MeO | MeO | 3-MeO | Br | 2{12} | 79 | 3{8} | 63 |
| 13 | MeO | MeO | 2-MeO | Br | 2{13} | 71 | 3{9} | 91 |
| 14 | OCH2O | 4-MeO | Br | 2{14} | 84b | 3{10} | 73 | |
| 15 | OCH2O | 2-MeO | Br | 2{15} | 83b | 3{11} | 63 | |
Isolated yields after column chromatography.
