Table 2.
Desketoraloxifene Analog Librarya
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| product 6/7 | R1 | R2 | R3 | NR4R5 | ion HRMS | HRMS (calcd) | HRMS (found) | purity (%)e | yield (%)f |
| 6{1} | H | H | H | piperidino | [M+H]+ | 413.1813 | 414.1894 | 98 | 83 |
| 6{2} | H | H | H | morpholino | [M+H]+ | 415.1606 | 416.1682 | >99 | 78 |
| 6{3} | H | H | H | pyrrolidino | [M+H]+ | 399.1657 | 400.1732 | 92 | 81h |
| 6{4} | H | H | H | NMe2 | [M+H]+ | 373.1500 | 374.1576 | 98 | 79 |
|
| |||||||||
| 6{5} | H | H | 4-MeO | piperidino | [M+H]+ | 443.1919 | 444.1987 | 98 | 83 |
| 7{1}b | H | H | 4-OH | piperidino | [M+H]+ | 429.1762 | 430.1835 | 98 | 27g |
| 6{6} | H | H | 4-MeO | morpholino | [M+H]+ | 445.1712 | 446.1788 | >99 | 73 |
| 7{2}b | H | H | 4-OH | morpholino | [M+H]+ | 431.1555 | 432.1636 | 75 | 23g |
| 6{7} | H | H | 4-MeO | pyrrolidino | [M]+ | 429.1762 | 429.1765 | 85 | |
| 7{3}b | H | H | 4-OH | pyrrolidino | [M+H]+ | 415.1606 | 416.1681 | 74 | 46g |
| 6{8} | H | H | 4-MeO | NMe2 | [M+H]+ | 403.1606 | 404.1673 | 98 | 81 |
| 7{4}b | H | H | 4-OH | NMe2 | [M+H]+ | 389.1449 | 390.1528 | 70 | 56 |
|
| |||||||||
| 6{9} | H | H | 3-MeO | piperidino | [M]+ | 443.1919 | 443.1916 | 78 | |
| 7{5}b | H | H | 3-OH | piperidino | [M+H]+ | 429.1763 | 430.1843 | >99 | 53g |
| 6{10} | H | H | 3-MeO | morpholino | 73 | ||||
| 7{6}b | H | H | 3-OH | morpholino | [M+H]+ | 431.1555 | 432.1636 | >99 | 47 |
| 6{11} | H | H | 3-MeO | pyrrolidino | 76 | ||||
| 7{7}b | H | H | 3-OH | pyrrolidino | [M+H]+ | 415.1606 | 416.1682 | 98 | 35g |
| 6{12} | H | H | 3-MeO | NMe2 | 69 | ||||
| 7{8}b | H | H | 3-OH | NMe2 | [M+H]+ | 389.1450 | 390.1525 | >99 | 51g |
|
| |||||||||
| 6{13} | H | H | 2-MeO | piperidino | [M]+ | 443.1919 | 443.1917 | 76 | |
| 7{9}b | H | H | 2-OH | piperidino | [M+H]+ | 429.1763 | 430.1841 | 92 | 17g |
| 6{14} | H | H | 2-MeO | morpholino | 78 | ||||
| 7{10}b | H | H | 2-OH | morpholino | ndi | ||||
| 6{15} | H | H | 2-MeO | pyrrolidino | 67 | ||||
| 7{11}b | H | H | 2-OH | pyrrolidino | ndi | ||||
| 6{16} | H | H | 2-MeO | NMe2 | 71 | ||||
| 7{12}b | H | H | 2-OH | NMe2 | [M+H]+ | 389.1450 | 390.1525 | >99 | 12g |
|
| |||||||||
| 6{17} | MeO | H | 4-MeO | piperidino | [M+H]+ | 473.2025 | 474.2050 | 99 | 87 |
| 7{13}c | OH | H | 4-OH | piperidino | [M+H]+ | 445.1712 | 446.1787 | 99 | 38g |
| 6{18} | MeO | H | 4-MeO | morpholino | 74h | ||||
| 7{14}c | OH | H | 4-OH | morpholino | [M+H]+ | 447.1504 | 448.1579 | 87 | 56g |
| 6{19} | MeO | H | 4-MeO | pyrrolidino | [M]+ | 429.1762 | 429.1768 | 81 | |
| 7{15}c | OH | H | 4-OH | pyrrolidino | [M+H]+ | 431.1555 | 432.1638 | 86 | 16g |
| 6{20} | MeO | H | 4-MeO | NMe2 | 81 | ||||
| 7{16}c | OH | H | 4-OH | NMe2 | ndi | ||||
|
| |||||||||
| 6{21} | MeO | H | 3-MeO | piperidino | [M]+ | 473.2025 | 473.2031 | 86 | |
| 7{17}c | OH | H | 3-OH | piperidino | [M+H]+ | 445.1712 | 446.1790 | 94 | 48g |
| 6{22} | MeO | H | 3-MeO | morpholino | [M+H]+ | 475.1817 | 476.1890 | 97 | 83h |
| 6{23} | MeO | H | 3-MeO | pyrrolidino | 71 | ||||
| 6{24} | MeO | H | 3-MeO | NMe2 | [M+H]+ | 433.1712 | 434.1790 | 96 | 82 |
| 7{18}c | OH | H | 3-OH | NMe2 | [M+H]+ | 405.1399 | 406.1475 | 89 | 17g |
|
| |||||||||
| 6{25} | MeO | H | 2-MeO | piperidino | [M+H]+ | 473.2025 | 474.2095 | 99 | 87 |
| 7{19}c | OH | H | 2-OH | piperidino | [M+H]+ | 445.1712 | 446.1787 | 95 | 52 |
| 6{26} | MeO | H | 2-MeO | morpholino | 81h | ||||
| 6{27} | MeO | H | 2-MeO | pyrrolidino | [M+H]+ | 459.1868 | 460.1946 | 98 | 83 |
| 7{20}c | OH | H | 2-OH | pyrrolidino | ndi | ||||
| 6{28} | MeO | H | 2-MeO | NMe2 | [M]+ | 433.1712 | 433.1720 | 83 | |
| 7{21}c | OH | H | 2-OH | NMe2 | [M+H]+ | 405.1399 | 406.1475 | 93 | 42g |
|
| |||||||||
| 6{29} | MeO | H | 3,5-(MeO)2 | piperidino | [M]+ | 503.2130 | 503.2132 | 77 | |
| 7{22}d | OH | H | 3,5-(OH)2 | piperidino | [M+H]+ | 461.1661 | 462.1737 | 33 | 7g |
| 6{30} | MeO | H | 3,5-(MeO)2 | morpholino | 78 | ||||
| 6{31} | MeO | H | 3,5-(MeO)2 | pyrrolidino | 73 | ||||
| 6{32} | MeO | H | 3,5-(MeO)2 | NMe2 | 73 | ||||
| 7{23}d | OH | H | 3,5-(OH)2 | NMe2 | [M+H]+ | 421.1348 | 422.1362 | 82 | 21g |
|
| |||||||||
| 6{33} | H | MeO | 4-MeO | piperidino | [M+H]+ | 473.2025 | 474.2105 | 98 | 83 |
| 7{24}c | H | OH | 4-OH | piperidino | [M+H]+ | 445.1712 | 446.1793 | 97 | 78 |
| 6{34} | H | MeO | 4-MeO | morpholino | 78 | ||||
| 7{25}c | H | OH | 4-OH | morpholino | [M+H]+ | 447.1504 | 448.1585 | 55 | 43g |
| 6{35} | H | MeO | 4-MeO | pyrrolidino | 75 | ||||
| 7{26}c | H | OH | 4-OH | pyrrolidino | [M+H]+ | 431.1555 | 432.1633 | 13 | 38g |
| 6{36} | H | MeO | 4-MeO | NMe2 | 76 | ||||
| 7{27}c | H | OH | 4-OH | NMe2 | [M+H]+ | 405.1399 | 406.1471 | 30 | 47 |
|
| |||||||||
| 6{37} | H | MeO | 2-MeO | piperidino | [M]+ | 473.2025 | 473.2019 | 78 | |
| 7{28}c | H | OH | 2-OH | piperidino | [M+H]+ | 445.1712 | 446.1914 | 97 | 37g |
| 6{38} | H | MeO | 2-MeO | morpholino | 77 | ||||
| 7{29} | H | OH | 2-OH | morpholino | [M+H]+ | 447.1504 | 448.1712 | 97 | 49g |
| 6{39} | H | MeO | 2-MeO | pyrrolidino | 81 | ||||
| 7{30}c | H | OH | 2-OH | pyrrolidino | [M+H]+ | 431.1555 | 432.1703 | 92 | 35g |
| 6{40} | H | MeO | 2-MeO | NMe2 | 82 | ||||
| 7{31}c | H | OH | 2-OH | NMe2 | [M+H]+ | 405.1399 | 406.1538 | 99 | 31g |
|
| |||||||||
| 6{41} | MeO | MeO | 4-MeO | piperidino | [M+H]+ | 503.2130 | 504.2146 | 95 | 76 |
| 7{32}d | OH | OH | 4-OH | piperidino | [M+H]+ | 461.1661 | 462.1732 | >99 | 12g |
| 6{42} | MeO | MeO | 4-MeO | morpholino | 75h | ||||
| 7{33}d | OH | OH | 4-OH | morpholino | [M+H]+ | 463.1453 | 464.1471 | 97 | 53g |
| 6{43} | MeO | MeO | 4-MeO | pyrrolidino | [M]+ | 489.1974 | 489.1981 | 79 | |
| 7{34}d | OH | OH | 4-OH | pyrrolidino | ndi | ||||
| 6{44} | MeO | MeO | 4-MeO | NMe2 | 69 | ||||
| 7{35}d | OH | OH | 4-OH | NMe2 | ndi | ||||
|
| |||||||||
| 6{45} | MeO | MeO | 3-MeO | piperidino | [M]+ | 503.2130 | 503.2134 | 79 | |
| 7{36}d | OH | OH | 3-OH | piperidino | ndi | ||||
| 6{46} | MeO | MeO | 3-MeO | morpholino | 81h | ||||
| 6{47} | MeO | MeO | 3-MeO | pyrrolidino | [M]+ | 489.1974 | 489.1982 | 73 | |
| 6{48} | MeO | MeO | 3-MeO | NMe2 | [M]+ | 463.1817 | 463.1826 | 72 | |
|
| |||||||||
| 6{49} | MeO | MeO | 2-MeO | piperidino | [M+H]+ | 503.2130 | 504.2209 | 99 | 77 |
| 6{50} | MeO | MeO | 2-MeO | morpholino | 72 | ||||
| 7{37}d | OH | OH | 2-OH | morpholino | [M+H]+ | 463.1453 | 464.1527 | >99 | 17g |
| 6{51} | MeO | MeO | 2-MeO | pyrrolidino | 68 | ||||
| 7{38}d | OH | OH | 2-OH | pyrrolidino | ndi | ||||
| 6{52} | MeO | MeO | 2-MeO | NMe2 | 71 | ||||
| 7{39}d | OH | OH | 2-OH | NMe2 | ndi | ||||
|
| |||||||||
| 6{53} | OCH2O | 4-MeO | piperidino | [M]+ | 487.1817 | 487.1817 | 81 | ||
| 6{54} | OCH2O | 4-MeO | morpholino | [M+H]+ | 489.1610 | 490.1692 | >99 | 77h | |
| 6{55} | OCH2O | 4-MeO | pyrrolidino | [M+H]+ | 473.1661 | 474.1754 | 98 | 69 | |
| 6{56} | OCH2O | 4-MeO | NMe2 | 79 | |||||
|
| |||||||||
| 6{57} | OCH2O | 2-MeO | piperidino | [M+H]+ | 487.1817 | 488.1896 | 93 | 76 | |
| 6{58} | OCH2O | 2-MeO | morpholino | 63h | |||||
| 6{59} | OCH2O | 2-MeO | pyrrolidino | 69h | |||||
| 6{60} | OCH2O | 2-MeO | NMe2 | [M]+ | 447.1504 | 447.1512 | 76 | ||
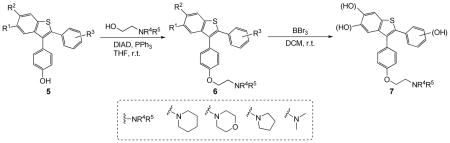
Reagents and conditions: i. Mitsunobu Coupling: 5 (0.2 mmol), alkylaminoethanol (1.5 equiv), DIAD (1.5 equiv), PPh3 (2.0 equiv), THF (2.0 mL), rt, 24–36 h. ii. Demethylation: 6 (0.1mmol), BBr3, CH2Cl2 (1.0 mL), rt, N2, 3 h.
2.0 Equiv of BBr3 used.
4.0 Equiv of BBr3 used.
6.0 Equiv of BBr3 used.
UV purity determined at 214 nm after preparative HPLC.
Isolated yields after column chromatography. All isolated products were characterized by 1H and13C NMR spectroscopy (see the Supporting Information).
Isolated yield after preparative HPLC.
An inseparable mixture was obtained.
The final product was not purified, because of poor solubility.
