Abstract
Quorum sensing (QS) is a population-dependent signaling process bacteria use to control multiple processes including virulence that is critical for establishing infection. The most common QS signaling molecule used by Gram-negative bacteria are acylhomoserine lactones. The development of non-native acylhomoserine lactone (AHL) ligands has emerged as a promising new strategy to inhibit QS in Gram-negative bacteria. In this work, we have synthesized a set of optically pure γ-lactams and their reduced cyclic azahemiacetal analogues, bearing the additional alkylthiomethyl substituent, and evaluated their effect on the AHL-dependent Pseudomonas aeruginosa las and rhl QS pathways. The concentration of these ligands and the simple structural modification such as the length of the alkylthio substituent has notable effect on activity. The γ-lactam derivatives with nonylthio or dodecylthio chains acted as inhibitors of las signaling with moderate potency. The cyclic azahemiacetal with shorter propylthio or hexylthio substituent was found to strongly inhibit both las and rhl signaling at higher concentrations while the propylthio analogue strongly stimulated the las QS system at lower concentrations.
Keywords: Azahemiacetals, Azasugars, Pseudomonas aeruginosa, Quorum sensing
INTRODUCTION
Quorum sensing (QS) is a type of bacterial cell-to-cell signaling pathway mediated through the production, release and detection of the small signaling molecules called autoinducers (AIs).1 Such communication allows bacterial control of crucial functions in united communities for enhancement of symbiosis, virulence, antibiotic production, biofilm formation, and many other processes. The recent increase in prevalence of bacterial strains resistant to antibiotics emphasizes the need for the development of a new generation of antibacterial agents. As QS is utilized by number of pathogenic bacteria to direct virulence and biofilm formation, inhibitors/modulators of QS may serve as tools to study or intercept such community behaviors and might be beneficial as antibacterial agents.2 The most common QS signaling molecule used by Gram-negative bacteria are acylhomoserine lactones (AHLs), which are detected by their cognate regulator (R) proteins.1
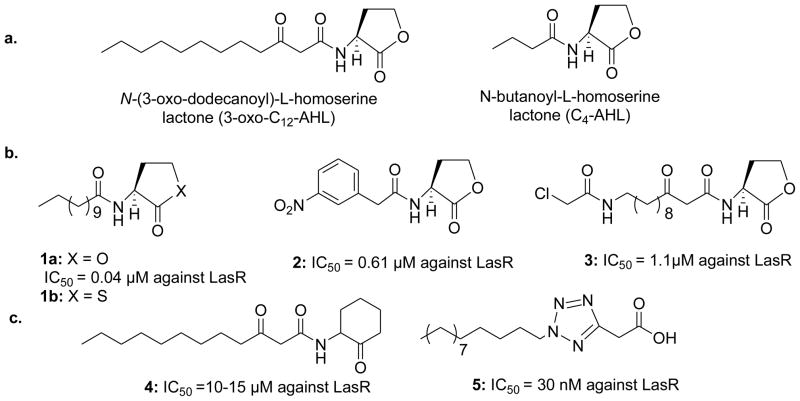
Pseudomonas aeruginosa is an important human opportunistic pathogen affecting immunocompromised individuals, cancer patients, burn victims, cystic fibrosis patients and patients with impaired lung function. It uses two AHL systems called, las and rhl to mediate QS. LasI/R synthesizes and detects N-(3-oxo-dodecanoyl)-L-homoserine lactone (3-oxo-C12-AHL) while RhlI/R synthesizes and detects N-butanoyl-L-homoserine lactone (C4-AHL) (Figure 1a). In addition, P. aeruginosa has a third QS-dependent pathway, Pseudomonas quinolone signal (PQS) that uses 2-heptyl-3-hydroxy-4-quinolone as an autoinducer.3 Although certain genes appear to be regulated by one pathway, for example regulation of genes involved in rhamnolipid synthesis by the rhl pathway,4 there is much overlap and crosstalk between the pathways and what was once thought to be hierarchical regulation, with las activating rhl, is now known to be much more complex.5 Accumulated evidences clearly indicate the importance of P. aeruginosa QS in disease.6
Figure 1.
(a) AHL-based signal molecules in P. aeruginosa. (b) Examples of potent synthetic QS inhibitors in various Gram-negative bacteria along with their reported IC50 values (tested in the same reporter strain used in the current studies).10,11 (c) Examples of QS inhibitors with a modified AHL scaffold tested in different reporter assays.12,13
Over two decades, several small molecules have been identified by many research groups as inhibitors of the AHL:R protein complex.7–9 These are mostly AHL-based structures with moderate changes on the acyl side chain and amide linkage. Some of the most potent inhibitors prepared by Geske and Blackwell (1a and 2, Figure 1b).10 Recently, Meijler and co-workers designed a ligand, 3, which covalently modified LasR.11 Since AHL is the pharmacophore present in the natural substrates, AHL-based inhibitors are likely to modulate R protein activation.
Studies of structural features other than the AHL scaffold as tools to understand the R type protein interaction with AHLs are limited,12 although the recent X-ray crystal structure of LasR with its native ligand and triphenyl mimcs ought to facilitate the rational design of QS inhibitors.14,15 Only a few examples of inhibitors with the altered lactone ring structure of AHL have been reported.12,13,16,17 For example, Smith et al. reported 3-oxo-C12-(2-aminocyclohexanone) (4, Figure 1c) as a strong antagonist of LasR system,12 while Muh et al identified two LasR inhibitors having a phenyl and tetrazole ring (e.g., 5), with IC50 in nM range.13 It is noteworthy that in the LuxR system γ-thiolactone analogue 1b showed inhibition while the corresponding ε-lactam (caprolactam) analogue was reported to lack LuxR binding.18 To explore further effects of non-native AHL scaffold on QS, we have designed novel lactam ligands. Here, we report optically pure γ-lactams and cyclic azahemiacetals, bearing alkylthiomethyl substituents with different carbon chain lengths (C3–C12), which are capable of either inhibiting or, in some cases, inducing P. aeruginosa QS pathways. The lactam ring was chosen because it is a more stable isoster of lactone ring present in AHL inhibitors. Moreover, the γ-lactam and cyclic azahemiacetal ligands were further modified in a such way that they resemble S-ribosyl-L-homocysteine, which is known to regulate QS through the LuxS-mediated biosynthesis of AI-2,1,19–21 in which the ribose oxygen is replaced with a nitrogen atom and the homocysteine unit is substituted with a simple alkylthiol chain. Although, P. aeruginosa does neither harbor LuxS nor produce AI-2, AI-2 does alter P. aeruginosa gene expression.22
RESULTS AND DISCUSSION
Design and synthesis
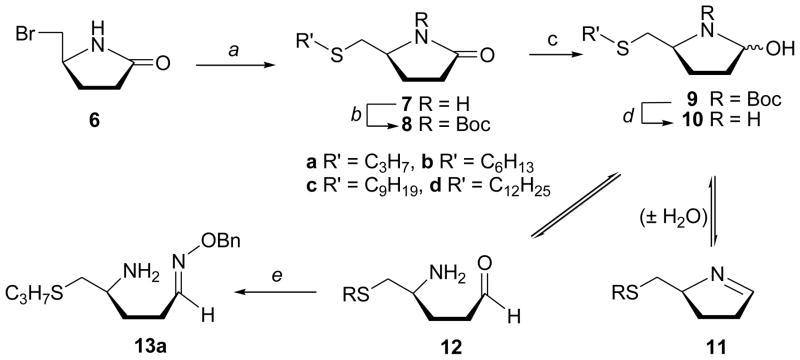
The (S)-5-(bromomethyl)pyrrolidin-2-one (6), a key substrate for the synthesis of γ-lactam analogue 7 was conveniently prepared from L-pyroglutamic acid.23 Displacement of bromide in 6 with sodium propanethiolate produced 5(S)-(propylthiomethyl)pyrrolidin-2-one (7a, 96%; Scheme 1). Since it was previously demonstrated that the length of the side chain is crucial for determining the agonistic and antagonistic activity,24 lactams containing C6, C9 and C12 alkylthio chain lengths (7b–d) were also analogously prepared. A set of the cyclic azahemiacetals (N,O-acetals or hemiaminals) 10 with a hydroxyl group instead of a carbonyl oxygen at C2 was synthesized as well. Thus, although, attempted reduction of lactams 7 with LiBEt3H was unsuccessful, reduction25 of the N-Boc protected lactams 8a–d proceeded smoothly to afford azahemiacetals 9a–d. Subsequent deprotection with trifluoroacetic acid afforded 5(S)-(alkylthiomethyl)pyrrolidin-2-ols 10a–d as a mixture of isomers in equilibrium25–28 with the corresponding imines 11a–d in addition to the open form aldehydes 12a–d. Structure of the hemiaminal 10a was additionally confirmed by conversion to the correponding O-benzyloxime derivative 13a with benzylhydroxylamine hydrochloride in anhydrous pyridine.
Scheme 1.
Reagents and conditions: (a) R'SH/NaH/DMF; (b) (Boc)2O/DMAP/CH2Cl2; (c) LiEt3BH/THF/CH2Cl2/−78 °C; (d )TFA/rt; (e) BnONH2 /pyr.
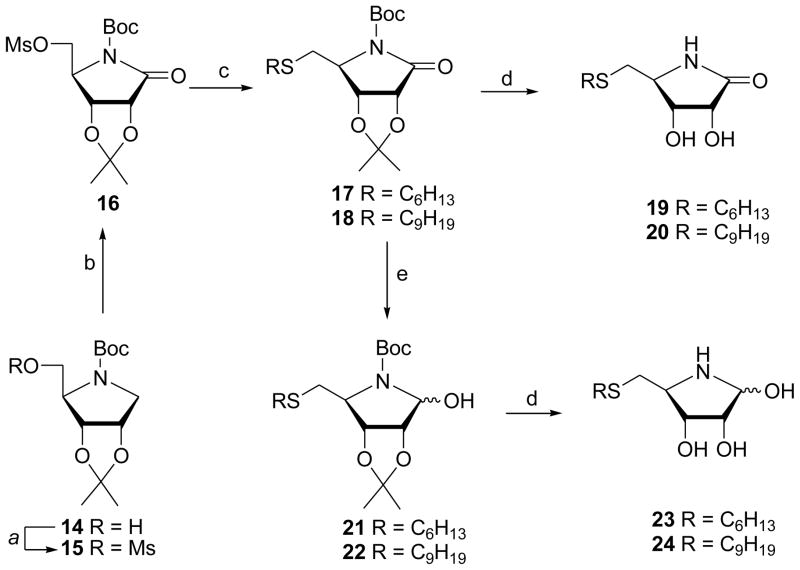
To increase the polarity/solubility of the lactam and azahemiacetal analogues in the testing media, we also prepared aza analogues with hydroxyl groups at C3 and C4. The N-Boc protected 1,4-dideoxy-1,4-imino-D-ribitol (14),29–31 conveniently prepared from D-gulonic-γ-lactone, served as a suitable starting material for the synthesis of dihydroxy γ-lactams 17 and 18. Thus, mesylation of ribitol 14 followed by the selective oxidation32 of the resulting 15 afforded 16. Displacement of the mesylate 16 with sodium hexane- and nonanethiolate produced 5-alkylthiomethyl lactams 17 and 18 in high yields which were deprotected with TFA to give (5S)-(hexyl- or nonylthiomethyl)-3,4-dihydroxypyrrolidin-2-ones 19 and 20 (Scheme 2). Reduction of 5-alkylthiomethyl lactams 17 and 18 with LiBEt3H afforded cyclic azahemiacetals 21 and 22. Subsequent deprotection with TFA provided (5S)-(alkylthiomethyl)-3,4-dihydroxypyrrolidin-2-ols 23 and 24 as a complex mixture of azahemiacetals existing in equilibrium with dehydrated form (imine) as well as with open aldehyde and dimeric forms, as reported for such class of 4-azaribofuranoses.25,28,33 These azahemiacetals can be considered as aza analogues of S-ribosyl-L-homocysteine28 (SRH) in which (a) ribose oxygen is replaced by nitrogen atom and (b) the homocysteine moiety is substituted with n-alkylthiols with different length of carbon chain.
Scheme 2.
Reagents and conditions: (a) MsCl/NEt3/CH2Cl2/rt; (b) NaIO4 /hydrated RuO2/EtOAc/H2O/rt; (C)RSH/NaH/DMF; (d )TFA/H2O; (e) LiEt3BH/THF/CH2Cl2 /− 78 °C.
Screening against las signaling
To determine the effect of the lactams (7, 19, and 20) and the cyclic azahemiacetal derivatives (10, 23 and 24) on the P. aeruginosa las AHL-mediated pathway, a las-dependent β-galactosidase reporter (PlasI-lacZ) was expressed with lasR in E. coli.34,35 Almost no activity was observed in absence of exogenous AHL demonstrating the AHL-dependence of the system (0.21 ± 0.31 Miller units). As expected, and in agreement with published data,34,35 addition of exogenous 3-oxo-C12-AHL activated the PlasI-lacZ (55.4 ± 1.5 Miller units). The lactams and their azahemiacetal counterparts were screened at a concentration of 0.2 to 1.2 mM for their activity against the las system (Table 1). At 0.29 mM concentration, the propylthio lactam 7a slightly but insignificantly inhibited LasR activity and its corresponding azahemiacetal 10a significantly stimulated (approximately by 2.3-fold) las reporter activity (p-value <0.01). This was dependent upon the addition of 2 μM of 3-oxo-C12-AHL as 10a did not stimulate β-galactosidase activity in the absence of exogenous AHL (data not shown). At the concentration of 0.57 mM, the azahemiacetal 10a was found to enhance las reporter activity by 15% (p-value <0.01). However, las reporter activity was inhibited by 69% (p-value <0.05) and 86% (p-value <0.01) at 0.87 and 1.14 mM, respectively. In contrast, the lactam analogue 7a inhibited las activity at all concentrations tested. Cell growth was not inhibited by the addition of lactam and azahemiacetal compounds at the tested concentrations (data not shown).
Table 1.
Effect of lactam and cyclic azahemiacetal derivatives on las and rhl systems.
| Compound | Concentration (mM) | Activitya (%) ± Standard Deviationb | Compound | Concentration (mM) | Activitya (%) ± Standard Deviationb | ||
|---|---|---|---|---|---|---|---|
| las | Rhl | las | rhl | ||||
| AHLc | 0.002 | 100 ± 0 | 100 ± 0c | ||||
|
| |||||||
| 7a | 0.29 | 90 ± 19 | 104 ± 24 | 10a | 0.29 | 228‡ ± 39 | 121* ± 6 |
| 0.58 | 72 ± 17 | 118 ± 0.8 | 0.57 | 115 ± 50 | 138* ± 3 | ||
| 0.87 | 51 ± 7 | 104 ± 13 | 0.86 | 31* ± 2 0 | 41† ± 1 | ||
| 1.16 | 43 ± 9 | 108 ± 17 | 1.14 | 11* ± 7 | 9‡ ± 1 | ||
|
| |||||||
| 7b | 0.23 | 106 ± 17 | 126 ± 8 | 10b | 0.23 | 109 ± 4 | 26 ± 0.2 |
| 0.47 | 87 ± 12 | 126 ± 29 | 0.46 | 0‡ ± 0 | 0.2† ± 0.3 | ||
| 0.70 | 74* ± 5 | 130 ± 31 | 0.69 | 0‡ ± 0 | 0‡ ± 0 | ||
| 0.93 | 61* ± 9 | 145‡ ± 19 | 0.92 | 0‡ ± 0 | 0* ± 0 | ||
|
| |||||||
| 7c | 0.19 | 72* ± 7 | 95 ± 3 | 10c | 0.19 | 74 ± 9 | 93 ± 12 |
| 0.39 | 26* ± 8 | 115 ± 30 | 0.39 | 65 ± 17 | 80 ± 13 | ||
| 0.58 | 18‡ ± 1 | 108 ± 12 | 0.58 | 57‡ ± 4 | 100 ± 11 | ||
| 0.78 | 6‡ ± 3 | 137 ± 46 | 0.77 | 47‡ ± 21 | 99 ± 3 | ||
|
| |||||||
| 7d | 0.17 | 52‡ ± 11 | 103 ± 7 | 10d | 0.17 | 72‡ ± 3 | 98 ± 13 |
| 0.33 | 32† ± 3 | 106 ± 6 | 0.33 | 56‡ ± 4 | 93 ± 27 | ||
| 0.50 | 18‡ ± 2 | 109 ± 8 | 0.50 | 45‡ ± 4 | 91 ± 8 | ||
| 0.67 | 11‡ ± 2 | 106 ± 5 | 0.66 | 34‡ ± 7 | 96 ± 5 | ||
|
| |||||||
| 19 | 0.20 | 96† ± 0.14 | 132* ± 36 | 23 | 0.20 | 84‡ ± 0.7 | 94 ± 8 |
| 0.40 | 78‡ ± 0.08 | 129 ± 10 | 0.40 | 68‡ ± 0.44 | 105 ± 3 | ||
| 0.61 | 79‡ ± 0.5 | 148 ± 9 | 0.60 | 51‡ ± 0.37 | 109 ± 12 | ||
| 0.81 | 68‡ ± 0.6 | 161‡ ± 7 | 0.80 | 42‡ ± 0.36 | 116 ± 18 | ||
|
| |||||||
| 20 | 0.17 | 93 ± 0.5 | 106 ± 3 | 24 | 0.17 | 78‡ ± 0.32 | 98 ± 5 |
| 0.35 | 87‡ ± 6 | 117‡ ± 8 | 0.34 | 78‡ ± 1 | 127* ± 14 | ||
| 0.52 | 80 ± 7 | 115* ± 11 | 0.52 | 71‡ ± 0.4 | 116 ± 9 | ||
| 0.69 | 63‡ ± 7 | 135‡ ± 5 | 0.69 | 53‡ ± 0.5 | 121 ± 14 | ||
The P. aeruginosa PlasI-lacZ and PrhlA-lacZ expressions in E. coli tested at the indicated concentrations (0.2–1.2 mM) against 2 μM of 3-oxo-C12-AHL or 2 μM of C4-AHL, respectively. The units are presented as percent activity relative to the DMSO treated control.
p-value < 0.05, †p-value < 0.02, ‡p-value < 0.01 according to a paired, two-tailed Student t-test. Statistically significant values are in bold.
The exogenous AHLs added to the las and rhl systems are 3-oxo-C12-AHL and C4-AHL, respectively. The 100% las and rhl activities refer to 55.4 ± 1.5 and 266 + 20 Miller units, respectively.
Among other lactam analogues tested, the percent inhibition of las promoter activity increased in a concentration dependent manner (Table 1). Inhibition potency also increased as the alkylthio chain length increased. Specifically, nonylthio lactam 7c and dodecylthio lactam 7d were found to possess greatest inhibition at all concentrations tested. At the lowest concentration tested (0.19 and 0.17 mM, respectively), nonylthio lactam 7c modestly inhibited while dodecylthio lactam 7d significantly inhibited las activity (48%; p-value <0.01). On the contrary, among the cyclic azahemiacetals analogues, no general trend was observed between chain length and percent inhibition. Unlike the propylthio azahemiacetal 10a, hexylthio azahemiacetal 10b did not significantly stimulate QS at the lowest concentration tested (0.23 mM) but inhibited las activity 100% at all higher concentrations used (p-value <0.01). In comparison, azahemiacetals containing nonyl side chain 10c and dodecyl chain 10d showed moderate but significant inhibitory activity (Table 1).
The ribolactam analogues 19 and 20 and their cyclic azahemiacetal counterparts 23 and 24 were found to significantly inhibit las activity at all concentrations tested but only with moderate potency. The azahemiacetals (23 and 24) appears slightly more active (Table 1).
Screening against the rhl pathway
To determine the effect of the lactams (7, 19, and 20) and the cyclic azahemiacetal derivatives (10, 23 and 24) on the P. aeruginosa rhl AHL-mediated pathways, a rhl-dependent β-galactosidase reporter (PrhlA-lacZ) was expressed with rhlR in E. coli.4 As expected, and in agreement with published data,4 exogenous C4-AHL activated the rhl β-galactosidase reporter (266 ± 20 Miller units). There was minimal activity in the absence of C4-AHL (0.12 ± 2.72 Miller units). In general, except for the cyclic azahemiacetals with shorter alkylthio chain 10a and 10b, the rest either had no effect or stimulated rhl expression (Table 1). The lactam analogues 7a, 7c and 7d did not modulate rhl activity, while hexylthio lactam 7b significantly stimulated (p-value <0.01) rhl QS activities at higher concentrations. In contrast to lactams, cyclic azahemiacetals with shorter alkylthio chain 10a (at higher concentration) and 10b (at all concentration) significantly inhibited rhl activity, while analogues 10c and 10d with longer alkyl chain were inactive. The hexylthio azahemiacetal 10b completely inhibited rhl signaling at concentrations of 0.46 mM and higher (p-value <0.02). The strong inhibition observed with propylthio 10a and hexylthio 10b azahemiacetal analogues having side chain lengths similar to C4-AHL is in agreement with the structure activity relationship reported for various synthetic AHL mimetics targeting RhlR.36
The hexyl-ribolactam analogue 19 significantly stimulated (p-value <0.01) rhl activity, while its cyclic azahmiacetal counterpart 23 had no effect (Table 1). The nonyl-azahemiacetal analogue 24 had significant stimulatory activity at 0.34 mM (p-value <0.05; Table 1).
CONCLUSIONS
We have designed and synthesized a set of optically pure γ-lactams with alkylthiomethyl substitution at carbon γ and their N,O-acetal counterparts. These ligands were evaluated for their effect on P. aeruginosa AHL-dependent las and rhl QS pathways isolated in E. coli. Lactam analogues 7 showed selectivity between two QS systems, acting as inhibitors against las signaling and weak activators against rhl signaling, possibly due to differences in the active sites of their cognate R proteins or transport of the native signaling molecule. Antagonism of las activity increased with the length of the alkylthio chain. Interestingly, the cyclic azahemiacetal derivative with shorter propylthio chain (10a) significantly stimulated las signaling at lower concentrations while strongly inhibiting both QS systems at higher concentrations. At least with 10a, the stimulation of the las system only occurs in the presence of exogenous AHL. It is possible that heterodimeric LasR is more active as compared to homodimers. The ribolactam (19 and 20) and cyclic azahemiacetal (24) analogs inhibited las and stimulated rhl moderately. Of all the compounds tested, the 5-(hexylthiomethyl)pyrrolidin-2-ol (10b) appears most potent inhibitor against both las and rhl systems. The mechanism of inhibition is still unknown. Since the effect tested utilized a whole cell assay, the QS inhibition could occur at multiple steps in the pathway. For example, the compound could affect the import of the natural ligand, compete with the natural ligand for binding to the regulator, LasR or RhlR, or alter binding of the QS regulator to the promoter. Alternatively, it is possible that the compounds with longer side chains affect the membrane and that the las pathway is more sensitive to these changes. Future experiments can address these possibilities. For example, quantification of extracellular AHL would reveal if the compounds affect AHL import. If this were the mechanism of action, it is expected that there would be less extracellular AHL in the absence of compound than in the presence. If the compound competes for binding to the regulator, inhibition should be overcome by increased AHL concentration. Given the central role the las and rhl QS pathways play in P. aeruginosa virulence, inhibitors such as the ones described here, have significant potential as therapeutics.
METHODS
General experimental methods are described in Supporting Information Section. The 1H and 13C NMR spectra were determined with solutions in CDCl3 unless otherwise noted
5-(Propylthiomethyl)pyrrolidin-2-one [7a(5S)]. Procedure A
Propanethiol (50 μL, 42 mg, 0.55 mmol) was added dropwise to a stirred suspension of NaH (35 mg, 0.875 mmol, 60%/mineral oil) in dry DMF (1 mL) under Ar atmosphere at 0°C. After 10 min (till gas evolution has ceased), solution of compound 623 [(5S), 82 mg, 0.46 mmol] in dry DMF (1 mL) was added dropwise, and after 15 min the reaction mixture was allowed to warm to ambient temperature. After 12 h the resulting mixture was quenched with water at 0°C, volatiles were evaporated, and the residue was column chromatographed (EtOAc → 10% MeOH/EtOAc) to give 7a(5S) (77 mg, 96%) as a colorless oil: 1H NMR δ 0.98 (t, J = 7.3 Hz, 3H), 1.60 (sx, J = 7.3 Hz, 2H), 1.76–1.87 (m, 1H), 2.25–2.34 (m, 1H), 2.34–2.45 (m, 2H), 2.52 (t, J = 7.3 Hz, 2H), 2.54 (dd, J = 7.7, 13.2 Hz, 1H), 2.68 (dd, J = 5.5, 13.2 Hz, 1H), 3.80 ('quint', J = 5.5 Hz, 1H), 6.73 (br. s, 1H); 13C NMR δ 13.4, 23.1, 26.6, 30.2, 34.7, 38.6, 53.9, 178.0; MS (APCI) m/z 174 (MH+). HRMS (AP-ESI) m/z calcd for C8H15NNaOS [M+Na]+ 196.0772; found 196.0779.
5-(Hexylthiomethyl)pyrrolidin-2-one [7b(5S)]
Treatment of 622 [(5S), 823 mg, 4.62 mmol] in dry DMF (6 mL) with a thiolate solution in dry DMF (6 mL) generated from hexanethiol (682 μL, 573 mg, 4.86 mmol), and NaH (204 mg, 5.09 mmol, 60%/mineral oil) by Procedure A [column chromatography (80% EtOAc/hexane → 5% MeOH/EtOAc)] gave 7b(5S) (932 mg, 94%) as a colorless oil: [α]D = +40.7 (c = 0.03, CHCl3); 1H NMR δ 0.90 (t, J = 7.0 Hz, 3H), 1.24–1.33 (m, 4H), 1.33–1.42 (m, 2H), 1.58 ('quint', J = 7.4 Hz, 2H), 1.78–1.87 (m, 1H), 2.27–2.46 (m, 3H) 2.53 (dd, J = 8.0, 13.4 Hz, 1H), 2.54 (t, J = 7.3 Hz, 2H), 2.70 (dd, J = 5.3, 13.2 Hz, 1H), 3.81 ('quint', J = 6.6 Hz, 1H), 6.47 (br. s, 1H); 13C NMR δ 14.0, 22.5, 26.8, 28.5, 29.7, 30.1, 31.4, 32.7, 38.7, 53.8, 177.7; MS (ESI) m/z 216 (100, MH+); HRMS (TOF MS-ESI) m/z calcd for C11H21NOSNa [M+Na]+ 238.1236; found 238.1252.
N-tert-Butoxycarbonyl-5-(propylthiomethyl)pyrrolidin-2-one [8a(5S)]. Procedure B
DMAP (114 mg, 0.93 mmol), and (Boc)2O (398 mg, 1.82 mmol) were added to a stirred solution of compound 7a (77 mg, 0.445 mmol) in CH2Cl2 (2 mL) at ambient temperature under Ar atmosphere. After 48 h, the reaction mixture was quenched with H2O (5 mL) and partitioned between CH2Cl2//NaHCO3/H2O. The organic layer was washed (brine), dried (MgSO4) and evaporated. The residue was column chromatographed (30 → 40% EtOAc/hexane) to give 8a (5S) (107 mg, 88%) as a colorless oil: 1H NMR δ 0.95 (t, J = 7.3 Hz, 3H), 1.50 (s, 9H), 1.58 (sx, J = 7.3 Hz, 2H), 1.96–2.04 (m, 1H), 2.06–2.17 (m, 1H), 2.40 (ddd, J = 2.6, 9.6, 17.9 Hz, 1H), 2.50 (“dt”, J = 4.9, 7.3 Hz, 2H), 2.58–2.67 (m, 1H), 2.60 (dd, J = 9.2, 13.5 Hz, 1H), 2.86 (ddd, J = 0.5, 2.8, 13.5 Hz, 1H), 4.20–4.27 (m, 1H); 13C NMR δ 13.3, 21.9, 23.1, 28.0, 31.2, 34.8, 35.4, 57.5, 83.1 , 149.8, 174.2; MS (ESI) m/z 274 (10, MH+), 215 (100, [MH-59]+).
N-tert-Butoxycarbonyl-5-(hexylthiomethyl)pyrrolidin-2-one [8b(5S)]
Treatment of 7b (311 mg, 1.45 mmol) in CH2Cl2 (6 mL) with DMAP (185 mg, 1.52 mmol), and (Boc)2O (746 mg, 3.42 mmol) by procedure B [column chromatography (20 → 40% EtOAc/hexane)] gave 8b(5S) (429 mg, 94%) as a colorless oil: 1H NMR δ 0.89 (t, J = 7.0 Hz, 3H), 1.25–1.33 (m, 4H), 1.34–1.42 (m, 2H), 1.55 (s, 9H), 1.59 ('quint', J = 7.4 Hz, 2H), 2.01–2.08 (m, 1H), 2.10–2.21 (m, 1H), 2.45 (ddd, J = 2.5, 9.6, 17.9 Hz, 1H), 2.56 ('dt', J = 2.9, 7.3 Hz, 2H), 2.62–2.72 (m, 1H), 2.63 (dd, J = 9.3, 13.5 Hz, 1H), 2.91 (dd, J = 2.7, 13.5 Hz, 1H), 4.24–4.31 (m, 1H); 13C NMR δ 14.0, 22.0, 22.5, 28.1, 28.4, 29.8, 31.2, 31.4, 32.9, 35.5, 57.5, 83.1, 149.8, 174.1; MS (ESI) m/z 315 (15, M+), 256 (100, [M-59]+); HRMS (TOF MS-ESI) m/z calcd for C16H29NO3SNa [M+Na]+ 338.1760; found 338.1752.
5-(Propylthiomethyl)pyrrolidin-2-ol [10a(5S)]. Step a. Procedure C
LiEt3BH (1M soln in THF, 0.98 mL, 0.98 mmol) was added to a stirred solution of 8a (107 mg, 0.39 mmol) in CH2Cl2 (3 mL) at −78 ºC under N2 atmosphere. After 30 min, the reaction mixture was quenched with MeOH (4 mL) and was allowed to warm to ambient temperature. Volatiles were evaporated and the residue was partitioned (EtOAc//NaHCO3/H2O), washed (brine) and dried (MgSO4). The resulting oil was chromatographed (30 → 40% EtOAc/hexane) to give N-tert-butoxycarbonyl-5-(propylthiomethyl)pyrrolidin-2-ol [9a(5S); 104 mg, 96%] as a colorless oil of the mixture of anomers/rotamers: MS (ESI) m/z 274 (10, [M-1]+), 258 (100, [M-17]+). Step b. Procedure D. Compound 9a (104 mg, 0.37 mmol) in TFA (4.0 mL) was stirred at room temperature for 2 h. Volatiles were evaporated to give 10a (62 mg, 96%) as a light yellow oil of a mixture of isomers accompanied by ~25% of the aldehyde 12a [1H NMR δ 8.89 (s, ~0.25H); and 13C NMR δ 180.8]; MS (ESI) m/z 158 (100, [M-17]+).
A solution of crude 10a (5S; 8 mg, 0.046 mmol) and O-benzylhydroxylamine hydrochloride (48 mg, 0.3 mmol) in anhydrous pyridine (1 mL) was stirred under an atmosphere of nitrogen at room temperature for 12 h. Pyridine was evaporated to afford 4-amino-5-(propylthio)pentanal O-benzyloxime [13a(4S)] of sufficient purity (~90%) for spectroscopic characterization together with the excess of BnONH2 used: MS (ESI) m/z 281 (60, MH+), 158 (100, [M-BnONH]+), (APCI) m/z 281 (100, MH+).
5-(Hexylthiomethyl)pyrrolidin-2-ol [10b(5S)]
Step a. Treatment of 8b (178 mg, 0.56 mmol) in CH2Cl2 (3 mL) with LiEt3BH (1M soln in THF, 1.41 mL, 1.41 mmol), by procedure C [quenched with MeOH (4 mL) at low temp., column chromatography (30 → 40% EtOAc/hexane)] gave N-tert-butoxycarbonyl-5-(hexylthiomethyl)pyrrolidin-2-ol [9b(5S); 170 mg, 95%)] as a colorless oil of a mixture of isomers: MS (ESI) m/z 316 (100, [M-1]+), 300 (20, [M-17]+); HRMS (TOF MS-ESI) m/z calcd for C16H31NO3SNa [M+Na]+ 340.1926; found 340.1955. Step b. Compound 9b (38.5 mg, 0.12 mmol) in TFA (0.8 mL) was stirred at 0 ºC (ice-bath) for 3 h. The reaction mixture was diluted with excess of ice-cold CH2Cl2 and neutralized with solid NaHCO3. Resulting mixture was stirred for 20 min at ambient temperature and was decanted. The residual slurry was extracted with fresh portion of CH2Cl2 and the combined extracts were dried (Na2SO4) and concentrated to give crude 10b (25 mg) as a colorless oil. Crude product was column chromatographed to give first (0 → 0.25% MeOH/CHCl3) anomeric mixture of azahemiacetals 10b (α/β, 9:20; 2.0 mg, 8%) as a colorless oil: 1H NMR δ 0.89 (t, J = 7.0 Hz, 4.35H), 1.26–1.45 (m, 8.7H), 1.47–1.72 (m, 3.9H), 1.75–1.85 (m, 0.45H), 1.92–2.07 (m, 3.45H), 2.10–2.23 (m, 0.9H), 2.46 (dd, J = 9.5, 13.0 Hz, 1H), 2.52–2.65 (m, 2.9H), 2.93–3.02 (m, 0.9H), 3.23 (dd, J = 2.5, 13.0 Hz, 1H), 3.60–3.68 (m, 1H), 3.67–3.75 (m, 0.45H), 4.04–4.10 (m, 1H), 4.20–4.27 (m, 0.45H); MS (ESI) m/z 200 (100, [M-17]+). Further elution (0.25 → 0.5% MeOH/CHCl3) gave imine 11b (5.8 mg, 22%) as a colorless oil: 1H NMR δ 0.91 (t, J = 7.0 Hz, 3H), 1.27–1.46 (m, 7H), 1.55–1.65 (m, 2H), 2.02–2.11 (m, 1H), 2.49–2.63 (m, 5H), 2.95 (dd, J = 5.3, 12.8 Hz, 1H), 4.21–4.29 (m, 1H), 7.63 (br t, J = 1.1 Hz, 1H); 13C NMR δ 14.0, 22.5, 26.1, 28.6, 29.8, 31.4, 33.0, 37.0, 38.1, 72.9, 167.0; MS (ESI) m/z 200 (100, MH+).
Note: The composition of crude products after step b depends strongly on the work up conditions. For example, the reaction mixture contained also ~22% of the aldehyde 12b [1H NMR δ 8.91 (s, ~0.22H)] at pH lower than 7.
4-Amino-N-(tert-butoxycarbonyl)-4-deoxy-2,3-O-isopropylidene-5-O-methanesulfonyl -D-ribono-1,4-lactam (16)
Step a. Triethylamine (93 μL, mg, 67 mg, 0.66 mmol) and MsCl (25 μL, 38 mg, 0.33 mmol) were added dropwise to stirred solution of 1430 (60 mg, 0.22 mmole) in anhydrous CH2Cl2 (6 mL) at 0 ºC (ice-bath). After 5 min, ice-bath was removed and the reaction mixture was allowed to stir at ambient temperature for 30 min. The reaction mixture was quenched with saturated NaHCO3/H2O and was extracted with CH2Cl2. The organic layer was washed (brine), dried (MgSO4) and evaporated to give 1-amino-1,4-anhydro-N-tert-butoxycarbonyl-1-deoxy-2,3-O-isopropylidene-5-O-methanesulfonyl-D-ribitol 15 (73 mg, 96%) as a mixture (~3:2) of two rotamers of sufficient purity to be directly used for next step: 1H NMR δ1.28 (s, 3, CH3), 1.42 (s, 12H, t-Bu, CH3), 2.96 (s, 1.2, Ms), 2.98 (s, 1.8, Ms), 3.39 (dd, J = 12.5, 4.8 Hz, 0.4H), 3.46 (dd, J = 12.5, 4.8 Hz, 0.6H), 3.69 (d, J = 12.5 Hz, 0.6H), 3.82 (d, J = 12.5 Hz, 0.4H), 4.10–4.14 (m, 0.4H), 4.22–4.30 (m, 1H), 4.22–4.29 (m, 1.4H), 4.45 (dd, J = 10.1, 4.1 Hz, 0.6H), 4.65 ('d', J = 5.9 Hz, 1H); 4.72 ('t', J = 5.3 Hz, 1H); 13C NMR (major) δ 24.9, 26.9, 29.6, 37.1, 52.5, 62.4, 68.9, 79.2, 80.4, 81.7, 112.1, 154.2; 13C NMR (minor) δ 24.9, 26.9, 29.6, 37.5, 53.1, 62.6, 68.6, 78.5, 80.6, 82.5, 112.1, 153.6; MS (APCI) m/z 352 (10, MH+), 252 (100, [MH2-Boc]+). Step b. RuO2×H2O (8.5 mg, 0.064 mmol) was added to a stirred solution of NaIO4 (172 mg, 0.96 mmol) in H2O (1 mL) at ambient temperature. After 5 min, a solution of 15 (80 mg, 0.32 mmol) in EtOAc (1 mL) was added dropwise and the reaction mixture was continued to stir for 12 h. H2O (20 mL) and EtOAc (20 mL) were added and the separated aqueous layer was furthermore extracted with EtOAc (2 × 20 mL). The combined organic layers were washed (brine), dried (MgSO4) and evaporated. The residue was column chromatographed (EtOAc) to give 16 (78 mg, 95%) as a colorless oil: 1H NMR δ 1.37 (s, 3H), 1.44 (s, 3H), 1.54 (s, 9H), 3.01 (s, 3H), 4.39–4.43 ('m', 2H), 4.58 (d, J = 5.45 Hz, 1H), 4.64 (dd, J = 11.2, 3.1 Hz, 1H), 4.70 (d, J = 5.45 Hz, 1H); 13C NMR δ 25.6, 27.0, 28.0, 37.7, 59.2, 67.0, 74.5, 77.5, 84.7, 112.8, 149.7, 170.2; MS (APCI) m/z 298 (100, [MH2-Boc+MeOH]+).
4-Amino-N-(tert-butoxycarbonyl)-4-deoxy-5-S-hexyl-2,3-O-isopropylidene-5-thio-D-ribono-1,4-lactam (17)
Treatment of 16 (60 mg, 0.16 mmol] in dry DMF (0.5 mL) with sodium hexathiolate [generated from hexanethiol (46.8 μL, 0.33 mmol)/NaH (14 mg, 0.35 mmol, 60%/mineral oil) in dry DMF (0.5 mL)] by Procedure A [column chromatography (5% → 10% MeOH/EtOAc)] gave 17 (25 mg, 40%) as a colorless oil and N-Boc deprotected 17 (24 mg, 38%) as a white crystalline solid. Compound 17 had: 1H NMR δ 0.81 (t, J = 7.0 Hz, 3H), 1.16–1.27 (m, 6H), 1.30 (s, 3H), 1.39 (s, 3H ), 1.44–1.59 (m, 11H), 2.36–2.50 (m, 2H), 2.76 (dd, J = 6.2, 14.4 Hz, 1H), 2.82 (dd, J = 2.7, 14.4 Hz, 1H), 4.31 (dd, J = 2.7, 6.2 Hz, 1H), 4.38 (d, J = 5.5 Hz, 1H), 4.78 (d, J = 5.5 Hz, 1H); 13C NMR δ 14.0, 22.5, 25.5, 27.0, 28.0, 28.3, 29.6, 31.3, 33.7, 33.9, 60.8, 76.1, 77.6, 83.9, 112.3, 149.8, 171.0; MS (APCI) m/z 288 (100, [MH2-Boc]+). N-Boc deprotected 14 had: 1H NMR δ 0.88 (t, J = 7.0 Hz, 3H), 1.25–1.36 (m, 6H), 1.38 (s, 3H), 1.48 (s, 3H), 1.56.–1.62 (m, 2H), 2.52.–2.75 (m, 3H), 2.73 (dd, J = 5.9, 13.4 Hz, 1H), 3.81 ('t', J = 6.1 Hz, 1H), 4.50 (d, J = 5.9 Hz, 1H), 4.69 (d, J = 5.9 Hz, 1H), 5.94 (s, 1H); 13C NMR δ 14.0, 29.7, 22.5, 28.5, 31.4, 25.6, 26.9, 33.2, 33.7, 58.0, 76.6, 79.2, 112.7, 173.2; MS (APCI) m/z 288 (100, MH+).
4-Amino-4-deoxy-5-S-hexyl-5-thio-D-ribono-1,4-lactam (19)
TFA/H2O (1 mL, 9:1) was added to 17 or N-Boc deprotected 17 (22 mg, 0.07 mmol) and the resulting solution was stirred at 0 ºC for 3 h. Evaporation of volatiles gave light yellow oil that was column chromatographed (5 → 10% MeOH/EtOAc) to give 19 (12 mg, 63%) as a colorless oil: 1H NMR δ 0.87 (t, J = 7.0 Hz, 3H), 1.24–1.39 (m, 6H), 1.52–1.59 (m, 2H), 2.50–2.55 (m, 3H), 2.73 (dd, J = 5.5, 13.6 Hz, 1H), 3.71 ('t', J = 6.4 Hz, 1H), 4.21 (d, J = 5.0 Hz, 1H), 4.44 (d, J = 5.0 Hz, 1H), 7.11 (s, 1H); 13C NMR δ 14.0, 29.6, 14.1, 22.5, 31.4, 32.7, 35.3, 59.9, 69.8, 71.8, 176.0; MS (APCI) m/z 248 (100, MH+); HRMS (TOF MS-ESI) m/z calcd for C11H21NO3SNa [M+Na]+ 270.1134; found 270.1137.
4-Amino-4-deoxy-5-S-hexyl-5-thio-α/β-D-ribofuranose (23)
Treatment of 17 (40 mg, 0.1 mmol) in THF (1 mL) with LiEt3BH (1M/THF, 0.26 mL, 0.26 mmol), by procedure C [column chromatography (10 → 20% EtOAc/hexane)] gave 4-amino-N-(tert-butoxycarbonyl)-4-deoxy-5-S-hexyl-3,4-O-isopropylidene-5-thio-α/β-D-ribofuranose 21 (39 mg, 97%)] as a colorless oil of the mixture of isomers: MS (ESI) m/z 389 (100, M+); HRMS (TOF MS-ESI) m/z calcd for C19H35NO5SNa [M+Na]+ 412.2128; found 412.2117. Deprotection of 21 (39 mg, 0.1 mmol) with TFA/H2O (0.9:0.1 mL) by Procedure D gave a light yellow oil that was column chromatographed (5 → 10% MeOH/EtOAc) to give 23 (22 mg, 88%) as a light yellow oil. 1H NMR showed a mixture of isomers accompanied by the open aldehyde form. MS (APCI) m/z 230 (40, MH+), 232 (100, [M-17]+).
Anti-Quorum Sensing Assay (β-Galactosidase Assay)
An overnight (O/N) culture of Escherichia coli DH5α harboring the plasmids pSC11, which contains a PlasI-lacZ translational fusion,34 and pJN105L, which contains a PBAD-lasR expression plasmid35 grown in LB media (10 g tryptone, 5 g yeast extract, 5 g sodium chloride per liter) supplemented with ampillicin (100 μg/ml) and gentamycin (15 μg/ml), was diluted to an OD600 of 0.150. At this time, arabinose (0.2 % w/v), N-3-(oxododecanoyl)homoserine lactone (3-oxo-C12-AHL; 2 μM), and either the compound under analysis or solvent (DMSO), was added to the culture (1.5 mL). A negative control containing only solvent, antibiotic, and arabinose (0.2 % w/v) without 3-oxo-C12-AHL was also assayed (data not shown). The cultures were incubated with shaking for three hours at 37 °C.
The conditions for the rhl biomonitor Escherichia coli DH5α harboring pECP61.5 plasmid, which contains Ptac-rhlR and PrhlA-lacZ4 were essentially same except that the LB medium was only supplemented with ampillicin (100 μg/ml), the O/N culture was diluted to an OD600 of 0.150, induced with 1 mM IPTG, 2 μM C4-HSL and the compounds or the controls added when the OD600 reached 1.0. A negative control containing only solvent, antibiotic and IPTG (1 mM) without C4-AHL was also assayed (data not shown). After incubation at 37°C for 4 hours with shaking, β-galactosidase activity was assayed as described previously.37 Miller units were calculated as described.38 Assays were repeated at least twice. For each biological replicate, experimental triplicates were performed and the average percent activity calculated by dividing the average Miller units from the samples containing compound or extract by the average Miller units from the sample containing solvent and multiplying by 100. Significance of inhibition was determined using a paired two-tailed Student t-test.
Supplementary Material
Acknowledgments
We thank NIGMS/NCI [SC1CA138176 (SFW) and 5R15AT002626 (KM)] and FIU’s Doctoral Evidence Acquisition Fellowship (VLM) for their support.
Footnotes
Supporting Information Available: General experimental methods, synthetic procedures and characterization data for compounds 7c–d, 8c–d, 10c–d, 18, 20, 22 and 24. This material is free via the Internet.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ng WL, Bassler BL. Annu Rev Genet. 2009;43:197. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raffa RB, Iannuzzo JR, Levine DR, Saeid KK, Schwartz RC, Sucic NT, Terleckyj OD, Young JM. J Pharmacol Exp Ther. 2005;312:417. doi: 10.1124/jpet.104.075150. [DOI] [PubMed] [Google Scholar]
- 3.Williams P, Camara M. Curr Opin Microbiol. 2009;12:182. doi: 10.1016/j.mib.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Pearson JP, Pesci EC, Iglewski BH. J Bacteriol. 1997;179:5756. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekimpe V, Deziel E. Microbiology. 2009;155:712. doi: 10.1099/mic.0.022764-0. [DOI] [PubMed] [Google Scholar]
- 6.Bjarnsholt T, Tolker-Nielsen T, Hoiby N, Givskov M. Expert Rev Mol Med. 2010;12:e11. doi: 10.1017/S1462399410001420. [DOI] [PubMed] [Google Scholar]
- 7.Ni N, Li M, Wang J, Wang B. Med Res Rev. 2009;29:65. doi: 10.1002/med.20145. [DOI] [PubMed] [Google Scholar]
- 8.Mattmann ME, Blackwell HE. J Org Chem. 2010;75:6737. doi: 10.1021/jo101237e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galloway WRJD, Hodgkinson JT, Bowden SD, Welch M, Spring DR. Chem Rev. 2011;111:28. doi: 10.1021/cr100109t. [DOI] [PubMed] [Google Scholar]
- 10.Geske GD, O'Neill JC, Miller DM, Mattmann ME, Blackwell HE. J Am Chem Soc. 2007;129:13613. doi: 10.1021/ja074135h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amara N, Mashiach R, Amar D, Krief P, Spieser SpAH, Bottomley MJ, Aharoni A, Meijler MM. J Am Chem Soc. 2009;131:10610. doi: 10.1021/ja903292v. [DOI] [PubMed] [Google Scholar]
- 12.Smith KM, Bu Y, Suga H. Chem Biol. 2003;10:81. doi: 10.1016/s1074-5521(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 13.Muh U, Schuster M, Heim R, Singh A, Olson ER, Greenberg EP. Antimicrob Agents Chemother. 2006;50:3674. doi: 10.1128/AAC.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou Y, Nair SK. Chem Biol. 2009;16:961. doi: 10.1016/j.chembiol.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zakhari JS, Kinoyama I, Struss AK, Pullanikat P, Lowery CA, Lardy M, Janda KD. J Am Chem Soc. 2011;133:3840. doi: 10.1021/ja111138y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morohoshi T, Shiono T, Takidouchi K, Kato M, Kato N, Kato J, Ikeda T. Appl Environ Microbiol. 2007;73:6339. doi: 10.1128/AEM.00593-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida T, Ikeda T, Takiguchi N, Kuroda A, Ohtake H, Kato J. Appl Environ Microbiol. 2007;73:3183. doi: 10.1128/AEM.02233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaefer A, Hanzelka B, Eberhard A, Greenberg E. J Bacteriol. 1996;178:2897. doi: 10.1128/jb.178.10.2897-2901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Nature. 2002;415:545. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 20.Gopishetty B, Zhu J, Rajan R, Sobczak AJ, Wnuk SF, Bell CE, Pei D. J Am Chem Soc. 2009;131:1243. doi: 10.1021/ja808206w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wnuk SF, Robert J, Sobczak AJ, Meyers BP, Malladi VLA, Zhu J, Gopishetty B, Pei D. Bioorg Med Chem. 2009;17:6699. doi: 10.1016/j.bmc.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan K, Dammel C, Stein J, Rabin H, Surette MG. Mol Microbiol. 2003;50:1477. doi: 10.1046/j.1365-2958.2003.03803.x. [DOI] [PubMed] [Google Scholar]
- 23.Otsuka M, Masuda T, Haupt A, Ohno M, Shiraki T, Sugiura Y, Maeda K. J Am Chem Soc. 1990;112:838. [Google Scholar]
- 24.Passador L, Tucker KD, Guertin KR, Journet MP, Kende AS, Iglewski BH. J Bacteriol. 1996;178:5995. doi: 10.1128/jb.178.20.5995-6000.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanardi F, Battistini L, Nespi M, Rassu G, Spanu P, Cornia M, Casiraghi G. Tetrahedron: Asymmetry. 1996;7:1167. [Google Scholar]
- 26.Zanardi F, Sartori A, Curti C, Battistini L, Rassu G, Nicastro G, Casiraghi G. J Org Chem. 2007;72:1814. doi: 10.1021/jo062406l. [DOI] [PubMed] [Google Scholar]
- 27.Xiang YG, Wang XW, Zheng X, Ruan YP, Huang PQ. Chem Commun. 2009;7045 doi: 10.1039/b915488d. [DOI] [PubMed] [Google Scholar]
- 28.Malladi VLA, Sobczak AJ, Meyer TM, Pei D, Wnuk SF. Bioorg Med Chem. doi: 10.1016/j.bmc.2011.07.043. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleet GWJ, Son JC. Tetrahedron. 1988;44:2637. [Google Scholar]
- 30.Murruzzu C, Riera A. Tetrahedron: Asymmetry. 2007;18:149. [Google Scholar]
- 31.Haidle AM, Myers AG. Proc Natl Acad Sci U S A. 2004;101:12048. doi: 10.1073/pnas.0402111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu XL, Qing FL. J Org Chem. 2005;70:3826. doi: 10.1021/jo050057+. [DOI] [PubMed] [Google Scholar]
- 33.Witte JF, McClard RW. Tetrahedron Lett. 1991;32:3927. [Google Scholar]
- 34.Chugani SA, Whiteley M, Lee KM, D'Argenio D, Manoil C, Greenberg EP. Proc Natl Acad Sci U S A. 2001;98:2752. doi: 10.1073/pnas.051624298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, Lequette Y, Greenberg EP. Molecular microbiology. 2006;59:602. doi: 10.1111/j.1365-2958.2005.04960.x. [DOI] [PubMed] [Google Scholar]
- 36.Geske GD, O'Neill JC, Blackwell HE. Chem Soc Rev. 2008;37:1432. doi: 10.1039/b703021p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathee K, Howe MM. J Bacteriol. 1990;172:6641. doi: 10.1128/jb.172.12.6641-6650.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller JH. Experiments in molecular genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor: 1972. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.