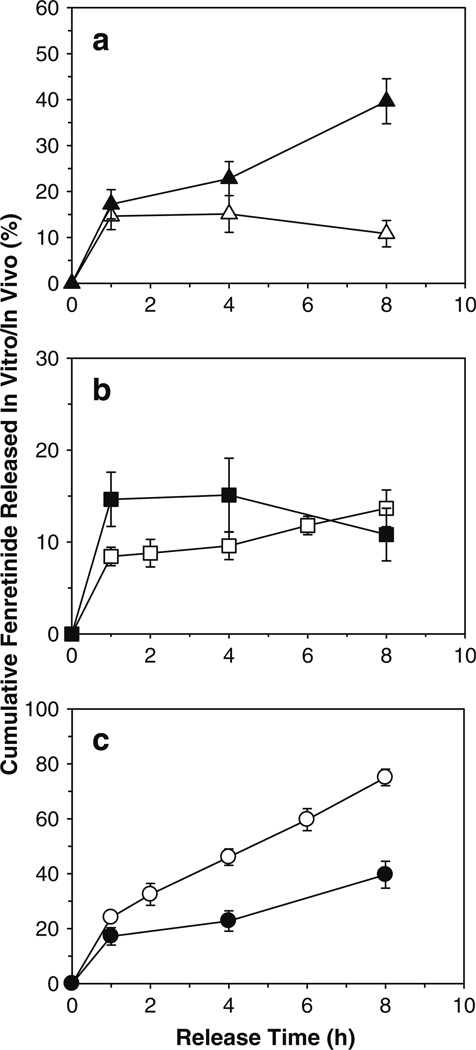
Fig. 6.
Co-incorporation of solubilizers provides significantly improved continuous release of fenretinide from Eudragit® RL PO mucoadhesive patches after buccal administration in rabbits. a Effect of co-incorporation of mixed solubilizers (0 (△) and 20 wt% Tween® 80 + 40 wt% sodium deoxycholate (▲)) on in vivo release of fenretinide. b Comparison of in vitro (□) and in vivo (■) fenretinide release from solubilizers-free patches. c Comparison of in vitro (○) and in vivo (●) fenretinide release from 20 wt% Tween® 80 + 40 wt% sodium deoxycholate-loaded patches. In vitro release studies were conducted in simulated saliva (pH 6.8) containing 5% w/v sodium deoxycholate at 37°C. Fenretinide loading (theoretical) in all patch formulations was 5 wt%. Solubilizer-free patch formulation was prepared using triethyl citrate (20 wt%) as a plasticizer. Solubilizer-loaded patches were prepared without triethyl citrate. Symbols represent mean ± SE, n = 3 (in vitro) or 6 (in vivo).