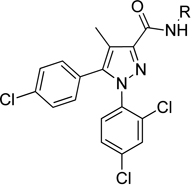
Table 2.
Sulfonamide and sulfamide derivatives 14–28 via Scheme 2
 | |||
|---|---|---|---|
| Compound | R | Ke CB1 (µM) | tPSA |
| 14 | 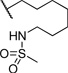 |
0.207 | 101 |
| 15 | 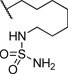 |
0.304 | 127 |
| 16 | >10 | 73 | |
| 17 | 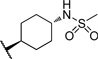 |
>10 | 101 |
| 18 | 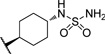 |
9.43 | 127 |
| 19a | 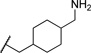 |
3.76 | 73 |
| 20a | 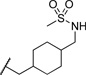 |
0.113 | 101 |
| 21a | 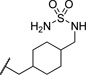 |
0.106 | 127 |
| 22b | 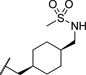 |
0.030 | 101 |
| 23b | 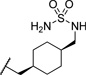 |
0.093 | 127 |
| 24 | 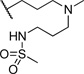 |
5.34 | 105 |
| 25 | 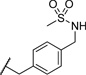 |
2.93 | 101 |
| 26 | 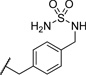 |
0.376 | 127 |
| 27 | 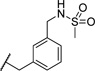 |
2.83 | 101 |
| 28 | 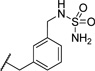 |
4.20 | 127 |
Compounds isolated are approximately 1:1 mixture of cis and trans isomers.
Compounds are 7:1 mixture of cis/trans isomers. A 7:1 mixture of 15 was isolated after careful column chromatography. Assignment of relative stereochemistry was tentatively made by H1NMR. Further efforts to isolate pure cis- and trans- isomers are ongoing.
