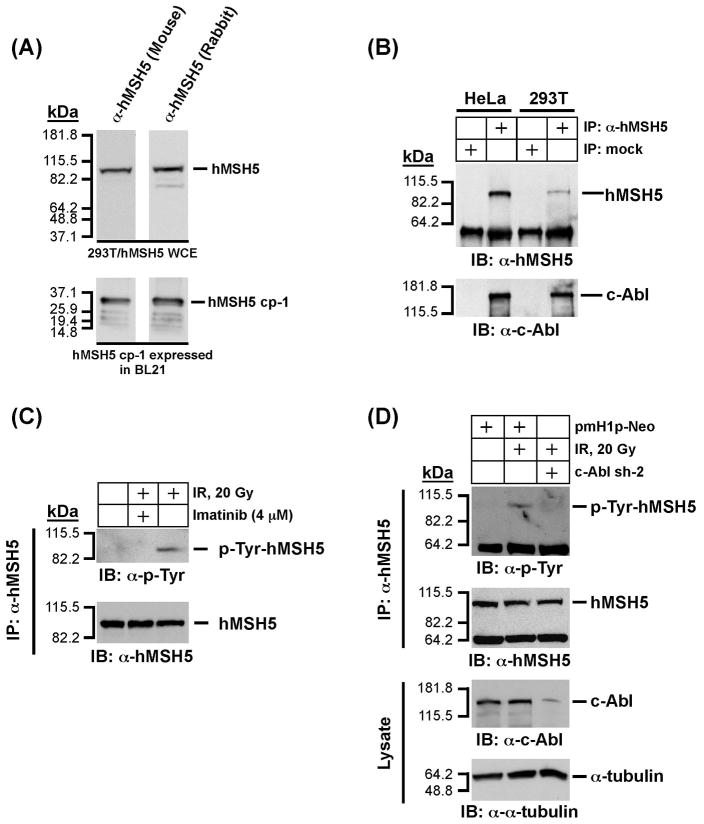
Figure 2.
Analysis of physical and functional interaction between endogenous hMSH5 and cAbl. (A) Characterization of a new mouse α-hMSH5 antiserum. Immunoblotting of 293T/hMSH5 whole cell extract (WCE) and partially purified bacterially expressed antigen hMSH5 cp-1 was performed with both the new mouse antiserum and the affinity-purified rabbit α-hMSH5 antibody. (B) Co-IP analysis of endogenous protein interaction between hMSH5 and c-Abl. Cell extracts prepared from approximately 0.1 ml of HeLa or 293T cell pellets were used to perform co-IP with 10 μl of the mouse α-hMSH5 antiserum. The presence of hMSH5 and cAbl in the immunoprecipitates was analyzed by Western blotting with α-hMSH5 and α-c-Abl antibodies. (C) The effect of c-Abl inhibition by imatinib on hMSH5 phosphorylation in response to IR in 293T cells. One hour after exposure to 20 Gy IR, cell extract was prepared and used to immunopurify hMSH5 with the mouse α-hMSH5 antibody. The status of hMSH5 tyro-sine phosphorylation was evaluated with an α-p-Tyr immunobot. To inhibit c-Abl activity, cells were pretreated with 4 uM imatinib 48 h prior to IR treatment. (D) Effect of c-Abl RNAi on IR-triggered hMSH5 phosphorylation. Cells were transfected with c-Abl sh-2 (or empty pmH1P-Neo vector as a control) at 48 h prior to IR treatment. The efficiency of c-Abl knockdown was validated with an α-c-Abl immunoblot, and its effect on IR-triggered hMSH5 phosphorylation was assessed by α-p-Tyr immunobotting. The α-tubulin blot was used as a loading control. kDa, molecular weight (Mr) in thousands.