Table 1.
Optimization of One-Pot Elimination and Double Aza-Michael Reactiona
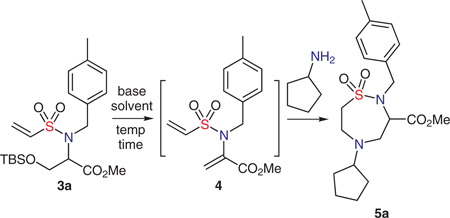 | ||||
|---|---|---|---|---|
| Entry | Base | Temp, time | Solvent | Yield (%)b |
| 1 | Cs2CO3 (1 equiv) | 40 °C, 12 h | MeCN | 50 |
| 2 | Cs2CO3 (1 equiv) | 40 °C, 12 h | CH2Cl2 | 44 |
| 3 | Cs2CO3 (1 equiv) | 40 °C, 12 h | MeOH | 0 |
| 4 | Cs2CO3 (1 equiv) | 60 °C, 12 h | MeCN | 43 |
| 5 | Cs2CO3 (2 equiv) | 60 °C, 12 h | MeCN | 32 |
| 6 | Cs2CO3 (1 equiv) | 40 °C, 12 h | DMF | 42 |
| 7 | DBU (0.5 equiv) | r.t., 12 h | MeCN | 23 |
| 8 | DBU (0.1 equiv) | 40 °C, 1 h | DMF | 30 |
| 9 | DBU (0.1 equiv) | r.t., 12 h | MeOH | 69 |
| 10 | DBU (0.1 equiv) | 40 °C, 1 h | MeOH | 75 |
A solution of compound 3a (1 equiv) and cyclopentylamine (1.1 equiv) in solvent (0.1 M) was added base and stirred at the indicated temperature and time.
Isolated yields following silica gel chromatography.
