Figure 2.
Advances in murine genetic manipulation.
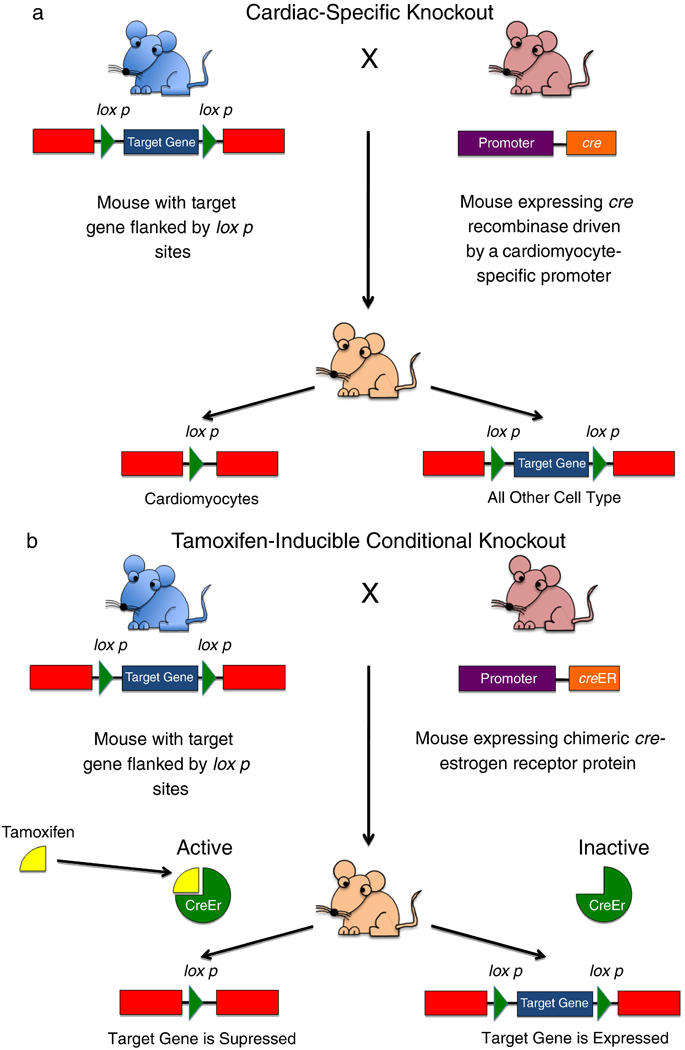
(a) Generation of a cardiac-specific gene knockout. The myocardium is a complex tissue, consisting of many cell types (myocytes, fibroblasts, vascular smooth muscle cells, vascular endothelial cells). Often it is desirable to remove a gene from one cell type only, to differentiate the role of each cell type in contributing to a specific cardiac pathology. This technology relies on the use of a DNA recombinase known as Cre (for cyclization recombination). This enzyme recognizes a specific 34 base pair-long site in DNA known as the loxP site. Cre induces a recombination event between two loxP sites, cutting out any DNA between them. One mouse is generated with the target gene of interest flanked by two loxP sites; this is known as a “floxed” gene (blue mouse). A second mouse is generated expressing Cre under the control of a cardiac-specific promoter, e.g. the α-myosin heavy chain (α-MHC) promoter (pink mouse). When these two mice are crossbred, their cells will contain both transgenes, however, Cre will be expressed only in cardiomyocytes, thus only in cardiomyocytes will the target gene be removed by the recombination event between the two loxP sites. All other cells will express that gene normally. (b) Generation of a conditional knockout. Sometimes it is desirable to remove a gene in a specific time frame. This is especially important when deletion of a gene from conception results in embryonic lethality, whereas deletion in the adult heart does not. In this case, a mouse is created with a floxed target gene (blue mouse). This mouse is crossbred to a mouse where Cre is fused to a mutated estrogen receptor which has lost its ability to bind endogenous hormones, but which can still bind tamoxifen. In the absence of tamoxifen, cre is maintained in an inactive state. However, when the mouse is given tamoxifen, it binds to the estrogen receptor which allows Cre to migrate to the nucleus and remove the target gene.