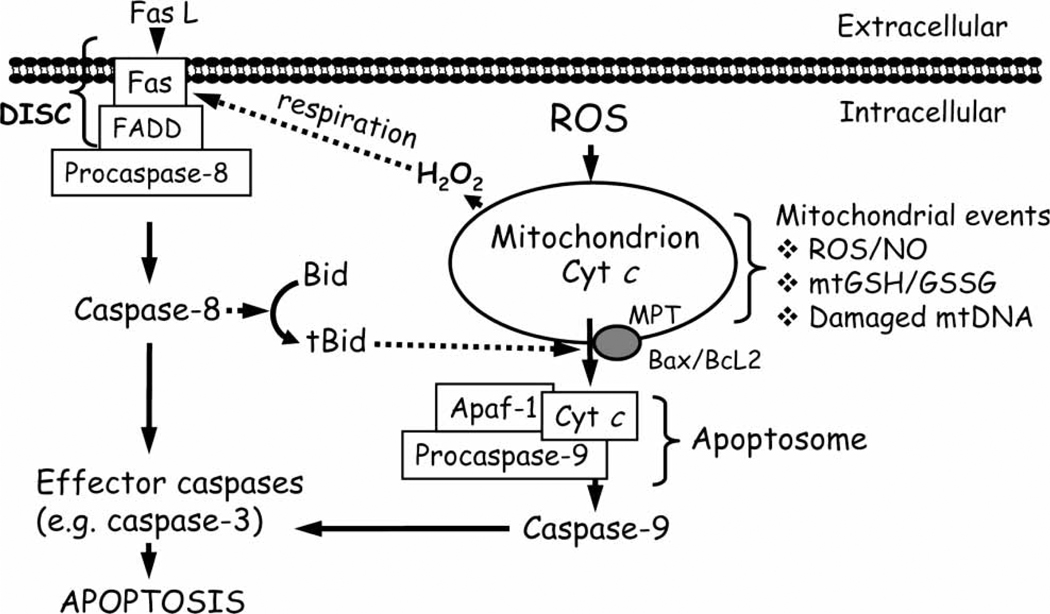
Figure 1.
Death receptor and mitochondria-mediated pathways of cellular apoptosis. Death receptor signalling is mediated by the binding of ligand, such as FasL to its membrane receptor and, together with the Fas-associated death domain (FADD), forms the death-inducing signalling complex (DISC). Subsequent activation of the initiator caspase-8 results in (A) direct activation of effector caspases, such as caspase-3 or (B) engagement of mitochondrial apoptotic signalling through cleavage of the pro-apoptotic protein, Bid. At the level of the mitochondrion, ROS/RNS; mtGSH/GSSG imbalance or an increase in mtDNA damage can stimulate apoptotic signalling which includes permeabilization of the mitochondrial membrane either via MPT opening or via pores formed by Bax and Bcl2 that results in mitochondria-to-cytosol release of apoptogenic factors such as cytochrome c (cyt c). Once in the cytosol, cyt c, together with pro-caspase-9 and Apaf-1 forms the apoptosome complex. Activated caspase-9 subsequently cleaves and activates downstream effector caspase-3 or -7, which mediates the final steps of apoptosis. FasL, Fas ligand; FADD, Fas-associated death domain; DISC, death-inducing signalling complex; Bid, BH3-only pro-apoptotic protein Bid; tBid, truncated form of Bid; Bax/Bcl2, pro- and anti-apoptotic proteins, respectively; cyt c, cytochrome c; Apaf-1, apoptotic protease activation factor-1; MPT, mitochondrial permeability transition; ROS, reactive oxygen species; NO, nitric oxide; mtGSH/GSSG, mitochondrial glutathione/glutathione disulphide; mtDNA, mitochondrial DNA.