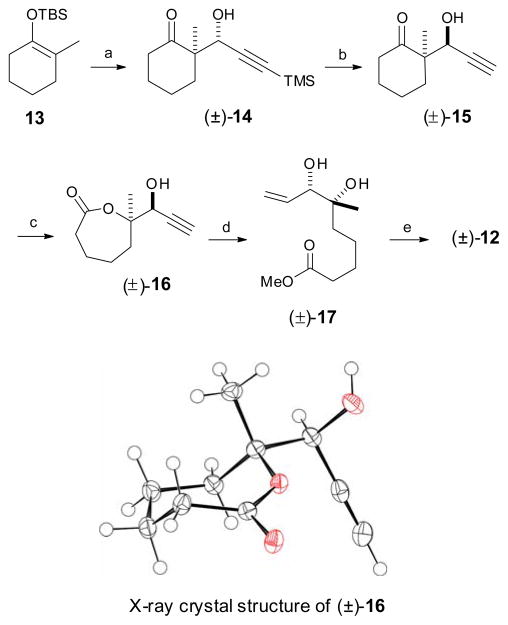
Scheme 2.
Synthesis of C1–C9 unit. Reagents and conditions: a) 3-trimethylsilylpropynal, BF3·OEt2, CH2Cl2, 60%; b) i. 4-NO2C6H4CO2H, TPP, DIAD, 40%; ii. K2CO3, MeOH, 70%; C) m-CPBA, NaHCO3; 4:1 Hexane: EtOAc, 70%; d) i. Et3N, MeOH, 90 °C, quant; ii. Lindlar catalyst, EtOAc:pyridine:1-octene, 97%; e) i. 2,2-dimethoxypropane, PPTS, 90%; ii. LiOH, 2:2:1 THF:MeOH:H2O, quant.