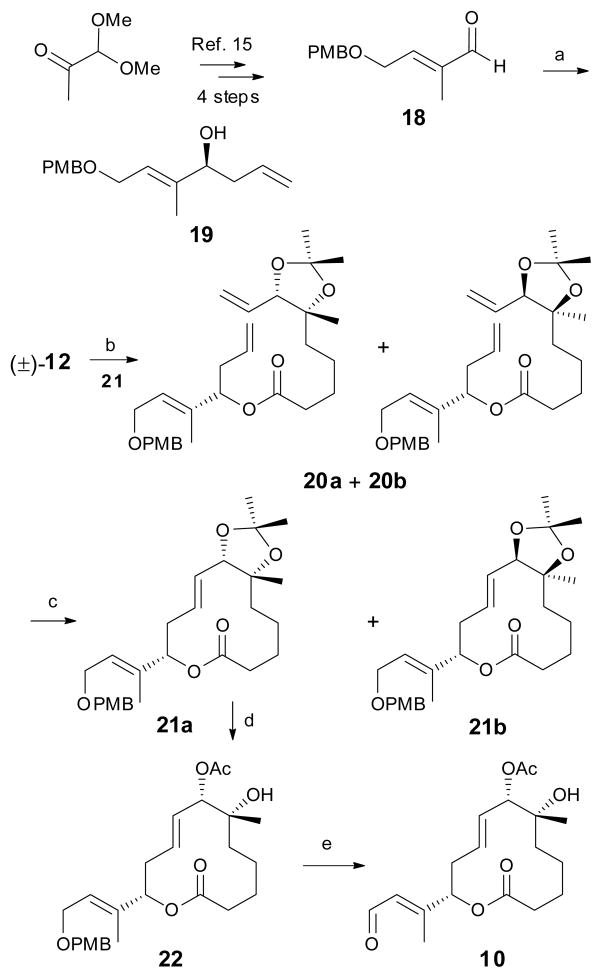
Scheme 3.
Synthesis of the C1–C14 unit. Reagents and conditions: a) (−)-Ipc2BOMe, Allylmagnesium bromide, diethyl ether, 89%; b) 19, 2,4,6-trichloro benzoylchloride, Et3N, DMAP, 88%, 1:1 diastereomers; c) 2nd generation Hoveyda-Grubbs catalyst, 49%, 3:2 diastereomers; d) i. PPTS, MeOH, 80 °C, 66%; ii. Et3N, Ac2O, DMAP, quant; e) i. DDQ, CH2Cl2, 70%; ii. Dess-Martin periodinane, CH2Cl2, 95%.