Figure 3.
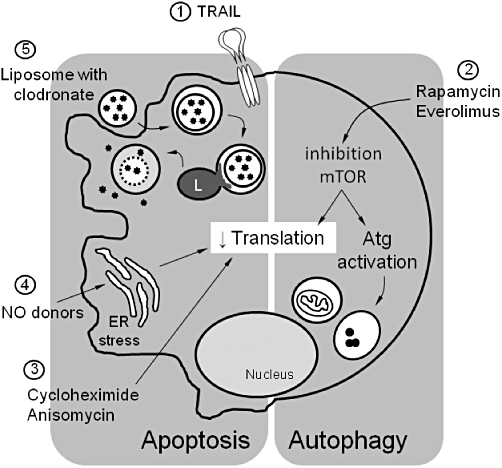
Overview of different strategies that induce selective macrophage death in atherosclerotic plaques. (1) TRAIL is expressed as a type II transmembrane protein, but its extracellular domain can be proteolytically cleaved from the cell surface and acts as a soluble cytokine interacting with transmembrane receptors belonging to the TNF-receptor family. Systemic soluble TRAIL selectively induces apoptosis of macrophages in atherosclerotic plaques. (2) Rapamycin and its structural analogue everolimus inhibit mammalian target of rapamycin (mTOR). Inhibition of mTOR downregulates translation and activates autophagy-related (Atg) genes. These effects may selectively induce autophagosome formation and autophagic cell death in macrophages of atherosclerotic plaques. (3) Inhibition of translation by the protein synthesis inhibitors cycloheximide or anisomycin selectively stimulates macrophage apoptosis in atherosclerotic plaques. (4) Treatment of atherosclerotic plaques with nitric oxide (NO) donors such as molsidomine depletes macrophages via apoptosis, potentially via induction of ER stress and subsequent inhibition of protein translation. (5) Liposomes encapsulating clodronate (or other lethal drugs) are ingested by macrophages via endocytosis. After fusion with lysosomes (L), the phospholipid bilayers of the liposomes are disrupted under the influence of lysosomal phospholipases. Clodronate is released into the cytosol and triggers apoptotic cell death. ER, endoplasmic reticulum; TRAIL, TNF-related apoptosis inducing ligand.
