Figure 2. Splicing of human K2P10.1 mRNA produces structurally different K+ channel subunits via alternative translation initiation (ATI).
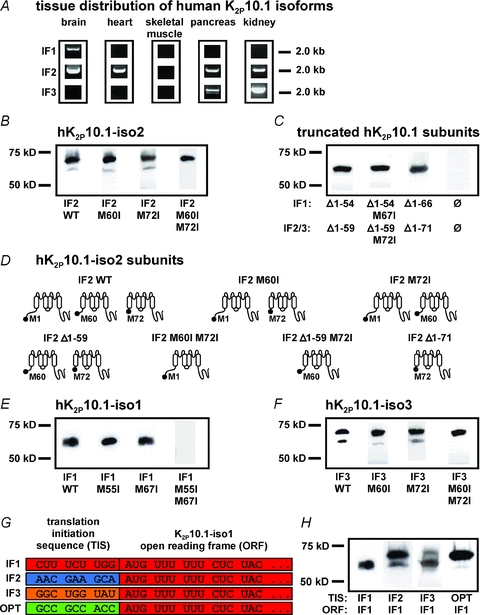
A, human tissue distribution of K2P10.1 splice variants (IF1, K2P10.1-iso1; IF2, K2P10.1-iso2; IF3, K2P10.1-iso3) assessed by PCR amplification of entire coding regions. B, Western blot analysis of hK2P10.1-iso2-1d4 wild-type (IF2 WT), hK2P10.1-iso2 M60I-1d4 (IF2 M60I), hK2P10.1-iso2 M72I-1d4 (IF2 M72I), or hK2P10.1-iso2 M60I M72I-1d4 (IF2 M60I M72I) subunits. C, protein analysis of indicated truncated hK2P10.1–1d4 subunits (see text for details). D, topology of hK2P10.1-iso2 protein variants arising from alternative mRNA translation initiation. E and F, Western blot analysis of oocytes expressing hK2P10.1-iso1-1d4 wild-type (IF1 WT; E), hK2P10.1-iso3-1d4 wild-type (IF3 WT; F), and indicated mutant cRNAs. G, recombination of translation initiation sequences (TIS). TIS from hK2P10.1 isoforms 1–3 (IF1–3) and artificial optimal TIS (OPT) preceded the open reading frame (ORF) of hK2P10.1-iso1-1d4 mRNA. H, translation initiation sequences differentially determine hK2P10.1-iso1 subunit expression. Western blots of proteins produced by expression of recombinant cRNAs described in panel G. K2P10.1 subunits were expressed in Xenopus oocytes and visualized with anti-1d4 antibody.
