Non-technical summary
The responsiveness of motoneurones within the spinal cord to non-invasive stimulation is markedly reduced during a fatiguing maximal effort if tested when voluntary drive from the brain is transiently interrupted. We tested a major possible cause of this effect by vibrating the tendon of the contracting muscle to increase excitatory input to the motoneurones. Application of tendon vibration had a negligible effect on the fatigue-induced reduction of motoneurone responsiveness. Hence, we believe the reduction in responsiveness is caused by changes to the intrinsic properties of motoneurones due to their repetitive activity during the sustained maximal effort.
Abstract
Abstract
Motoneurone excitability is rapidly and profoundly reduced during a sustained maximal voluntary contraction (MVC) when tested in the transient silent period which follows transcranial magnetic stimulation (TMS) of the motor cortex. One possible cause of this reduction in excitability is a fatigue-induced withdrawal of excitatory input to motoneurones from muscle spindle afferents. We aimed to test if muscle spindle input produced by tendon vibration would ameliorate suppression of the cervicomedullary motor-evoked potential (CMEP) in the silent period during a sustained MVC. Seven subjects performed a 2 min MVC of the elbow flexors. Stimulation of the corticospinal tract at the level of the mastoids was preceded 100 ms earlier by TMS. These stimulus pairs were delivered every 10 s during the 2 min MVC. Stimulus pairs at 30, 50, 70, 90 and 110 s were delivered while vibration (∼80 Hz) was applied to the distal tendon of biceps. On a separate day, the protocol was repeated with both stimuli delivered to the motor cortex. The CMEP in the silent period decreased rapidly with fatigue (to ∼9% of control) and was not affected by tendon vibration (P = 0.766). The motor-evoked potential in the silent period also declined rapidly (to ∼5% of control) and was similarly unaffected by tendon vibration (P = 0.075). These data suggest motoneurone disfacilitation due to a fatigue-related decrease of muscle spindle discharge does not contribute significantly to the profound suppression of motoneurone excitability during the silent period. Therefore, a change to intrinsic motoneurone properties caused by repetitive discharge is most probably responsible.
Introduction
Stimulation of the corticospinal tract at the level of the mastoids produces a single descending volley which can be recorded at the target muscle as a cervicomedullary motor-evoked potential (CMEP). As the stimulus is delivered subcortically, CMEPs are not affected by changes in cortical excitability but are sensitive to motoneurone excitability. Unlike the Ia afferent input which evokes H-reflexes, corticospinal–motoneuronal synapses lack classical presynaptic inhibition (e.g. Nielsen & Petersen, 1994; Rudomin & Schmidt, 1999; Jackson et al. 2006), but they may undergo activity-dependent synaptic changes (Gandevia et al. 1999; Petersen et al. 2003).
During the last 30 s of a sustained 2 min maximal voluntary contraction (MVC) CMEP size decreases, which suggests a reduction in motoneurone excitability (Butler et al. 2003; Martin et al. 2006). Interpretation of this reduction is difficult because it is unknown how the level of descending voluntary drive reaching the motoneurones is affected by the sustained maximal effort. Transcranial magnetic stimulation (TMS) of the motor cortex during a voluntary contraction transiently suppresses descending voluntary drive in what is termed the silent period. We recently used a paired-stimulus protocol (corticospinal stimulation delivered 100 ms after conditioning TMS) to test the effect of fatigue on CMEPs in the silent period during a sustained maximal effort. This showed that the CMEP was virtually abolished within 30 s in the absence of voluntary drive (McNeil et al. 2009). Our data argued against activity-dependent synaptic changes as the cause of the decrease and suggested a profound reduction in motoneurone excitability. Although we were unable to identify the precise mechanism(s) for this reduction, we proposed two possibilities: altered motoneurone properties due to repetitive discharge (e.g. Kernell & Monster, 1982) and motoneurone disfacilitation due to decreased muscle spindle discharge from fatigue-induced slowed muscle relaxation (Todd et al. 2005; see Fig. 1). Many lines of evidence from both animal (Eccles et al. 1957; Kirkwood & Sears, 1982; Heckman & Binder, 1988) and human studies (Magladery et al. 1951; De Gail et al. 1966; Macefield et al. 1993) have indicated the important net facilitation provided by primary muscle spindle endings to homonymous and heteronymous motoneurone pools.
Figure 1. An example of fatigue-related slowing of muscle relaxation rate and lengthening of the silent period recorded from a single subject during a sustained 2 min MVC.
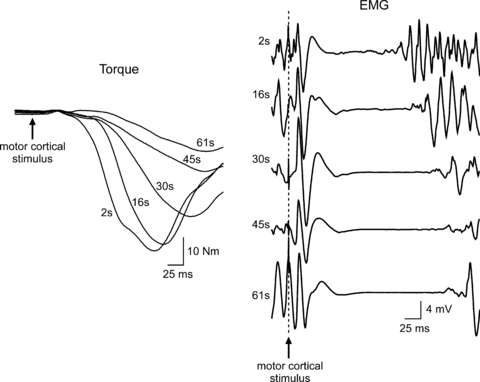
Responses were obtained to single transcranial magnetic stimulation during a 2 min sustained maximal effort. Torque responses demonstrate the progressive fatigue-related slowing of muscle relaxation. Normalised to the pre-stimulus torque to account for the loss of maximal torque production, the peak rate of relaxation slowed from −10.5 s−1 to −5.3 s−1 between 2 and 61 s. EMG responses demonstrate the progressive lengthening of the silent period (see Fig. 3 of McNeil et al. 2009 for group data). Traces were collected during the control experiment reported in McNeil et al. (2009).
The aim of this experiment was to test our previous suggestion that decreased muscle spindle afferent input contributed to the abolition of CMEPs in the silent period during a sustained maximal effort. We hypothesised that tendon vibration would increase spindle afferent input to the motoneurones and thereby delay the time course and magnitude of suppression of the CMEP in the silent period. The experiment was repeated with paired-TMS to assess if vibration affected motor cortical output.
Methods
Subjects
Seven healthy subjects (35 ± 12 years, mean ± SD; 3 females) participated in two protocols performed on separate days in a random order. All studies were approved by the University of New South Wales human research ethics committee and conformed to the Declaration of Helsinki. Written consent was obtained from each of the participants.
Experimental set-up
Subjects were seated with their right arm positioned in an isometric myograph and at an angle of ∼90 deg flexion at both the shoulder and elbow joints. The forearm was supinated and a strap at the wrist tightly secured the arm to the myograph. A linear strain gauge (Xtran, Melbourne, Australia) mounted to the myograph was calibrated to measure elbow flexor torque. Electromyographic activity (EMG) of biceps brachii was recorded via adhesive Ag–AgCl electrodes (10 mm diameter) positioned over the muscle belly and proximal portion of the distal tendon.
Torque and EMG data were recorded to computer using a 12-bit A/D converter (CED 1401 Plus; Cambridge Electronic Design Ltd, Cambridge, UK) and Spike2 software (version 6.06; Cambridge Electronic Design). The torque and EMG data were sampled at 1000 and 2000 Hz, respectively. EMG data were amplified (×100) and bandpass filtered (16–1000 Hz) using CED 1902 amplifiers (Cambridge Electronic Design).
Transcranial magnetic stimulation
A circular coil (13.5 cm outer diameter) attached via a BiStim unit to two Magstim 200 stimulators (Magstim, Dyfed, UK) was held over the vertex to stimulate the motor cortex. One stimulator delivered the conditioning stimulus (same intensity for both protocols; 88 ± 11% of stimulator output) and the other delivered the test stimulus (protocol B only; 56 ± 4% of stimulator output).
Cervicomedullary stimulation
In protocol A, the test stimulus was evoked by stimulation of the corticospinal tract with a high-voltage electrical current (100 μs duration, 200–350 mA, Digitimer DS7AH) passed between adhesive Ag–AgCl electrodes fixed to the skin over the mastoid processes (Ugawa et al. 1991; Gandevia et al. 1999). The CMEP recorded was termed unconditioned if the test stimulus was delivered on its own and conditioned if the test stimulus was delivered 100 ms after the conditioning TMS.
Vibration
To increase muscle spindle discharge and afferent feedback to the biceps motoneurone pool, vibration at a frequency of ∼80 Hz (Roll et al. 1989) was applied via a handheld pneumatic vibrator to the distal biceps tendon just distal to the reference EMG electrode at various stages of the experiment. Applied to the tendon with the muscle relaxed, vibration produced a tonic vibration reflex and an illusion of movement. An increase in muscle output (force) has been observed when tendon vibration is applied after fatigue has developed during a sustained MVC (Bongiovanni & Hagbarth, 1990). The erratic torque trace caused by the frequent stimuli used in this experiment made it difficult to consistently identify such a clear increase in torque. Hence, five of the subjects performed a control experiment which involved sustained MVCs but no stimulation. Subjects sustained an MVC and 10 s of vibration was applied when torque decreased by ∼50–60% (i.e. to a torque level which is comparable to that during most of the periods of vibration in the main experiment). Four to eight of these sustained MVCs were performed with short periods of rest between them. A clear effect of vibration was visible in all subjects and torque increased by an average of 4% across subjects. Raw traces from a single subject are shown in Fig. 2.
Figure 2. Examples of a vibration-related increase in elbow flexor torque recorded from a single subject during repeated sustained MVCs.
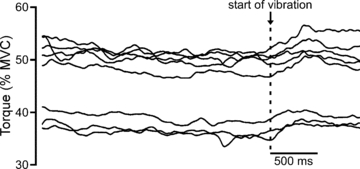
Vibration of the distal biceps tendon began when maximal voluntary torque decreased by ∼50–60% and is indicated by the dashed line. During these sustained efforts, torque increased by ∼4% within ∼250 ms of the onset of vibration.
Experimental procedures
Protocol A
Brief MVCs separated by 90 s of rest were performed to determine the intensities of the conditioning and test stimuli. Intensity of the conditioning TMS was set at the level which produced a silent period of ∼200 ms. Test stimulus intensity was adjusted to produce a conditioned CMEP comparable in size (∼4 mV) to our earlier experiment (McNeil et al. 2009). Using the desired levels of stimulation, six control unconditioned and conditioned CMEPs were collected during brief MVCs with at least 90 s of rest between efforts (Fig. 3). To assess if tendon vibration had an effect on conditioned CMEP size when the muscle was not fatigued, three of these unconditioned and conditioned CMEPs were collected with tendon vibration. Tendon vibration began ∼1 s prior to the MVC and continued for ∼1 s afterward. The use of vibration and single vs. paired stimuli was pseudo-randomised during this collection of control data. The sustained 2 min MVC began at least 90 s after the final control MVC. Strong verbal encouragement and visual feedback of elbow flexion torque were provided throughout the contraction to motivate subjects to maintain maximal effort. Beginning at 5 s, single test and paired stimuli were alternated every 5 s during the contraction. Vibration was applied for 10 s periods beginning at 22, 42, 62, 82 and 102 s. Raw traces showing conditioned CMEPs with and without vibration during the 2 min MVC are displayed for a single subject in Fig. 4.
Figure 3. Schematic diagram of protocol A.
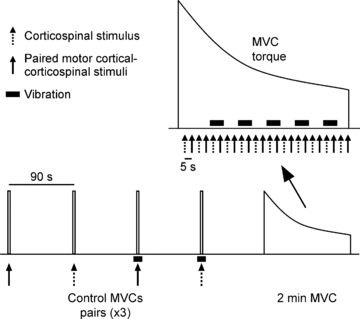
Twelve brief control MVCs were performed during which paired conditioning–test stimuli or a single test stimulus were delivered. Vibration (∼80 Hz) was applied to the distal biceps tendon on half of these efforts. Single and paired stimuli were delivered alternately at regular intervals whereas vibration was applied periodically during the sustained 2 min MVC. In protocol B, the test stimulus was a motor cortical rather than corticospinal stimulus.
Figure 4. Individual traces of CMEPs in the silent period recorded from a single subject during brief control MVCs and a sustained 2 min MVC.
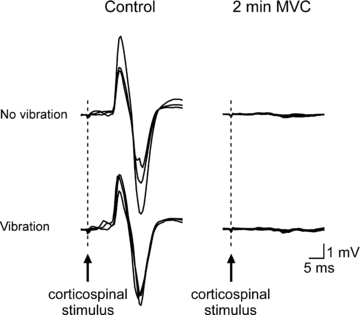
Mean traces of the three conditioned CMEPs with and without vibration are displayed in the left panel. Five responses obtained with (30, 50, 70, 90 and 110 s) and without (40, 60, 80, 100 and 120 s) vibration during the 2 min MVC are overlaid in the right panel. During the sustained effort, conditioned CMEPs were markedly reduced and of similar size both with and without vibration.
Protocol B
To assess the effect of tendon vibration on motor cortical responsiveness, TMS replaced electrical cervicomedullary stimulation as the test stimulus. Subjects completed the control and fatigue procedures as outlined in protocol A.
Data analysis and statistics
During off-line analysis, Signal software (version 4.07; Cambridge Electronic Design) was used to determine the area of unconditioned and conditioned CMEPs and MEPs. Unconditioned potentials were not affected by tendon vibration and will not be discussed further because the results are similar to those in our previous study (McNeil et al. 2009). Control values represent the means of the three potentials recorded with and without vibration during brief MVCs. To examine the effect of vibration during the sustained 2 min MVC, mean values were obtained for the five conditioned potentials collected with vibration (30, 50, 70, 90 and 110 s) and the last five conditioned potentials collected without vibration (40, 60, 80, 100 and 120 s). Separately for CMEPs and MEPs, paired t tests were used to compare the effect of vibration on conditioned area during control MVCs and then the sustained 2 min MVC. To confirm our previous conclusion that spinal mechanisms are responsible for the fatigue-induced suppression of the MEP (McNeil et al. 2009), another paired t test was used to compare conditioned CMEP area to MEP area during the sustained 2 min MVC (mean of all potentials from 30 s onward). Data are reported in the text as mean ± SD. The significance level was P < 0.05.
Results
Control measures prior to fatigue
During brief MVCs, vibration had no effect on the size of conditioned CMEPs (P = 0.343) or MEPs (P = 0.564). Conditioned CMEP amplitudes without and with vibration were 4.0 ± 1.4 and 3.5 ± 1.9 mV, respectively. Without and with vibration, conditioned MEP amplitudes were 4.0 ± 1.4 and 4.3 ± 1.7 mV, respectively.
Fatigue measures
During the sustained 2 min MVC, there was a rapid decrease of the conditioned CMEPs and MEPs (Fig. 5A) which was similar in both time course and magnitude to our previous result (McNeil et al. 2009). Intermittent bursts of tendon vibration did not affect the size of conditioned CMEPs (P = 0.766) or MEPs (P = 0.075) (Fig. 5A and B). MEPs tended to be suppressed more than CMEPs but this was not statistically significant (P = 0.080) (Fig. 5B).
Figure 5. Normalised responses in the silent period during a sustained 2 min MVC.
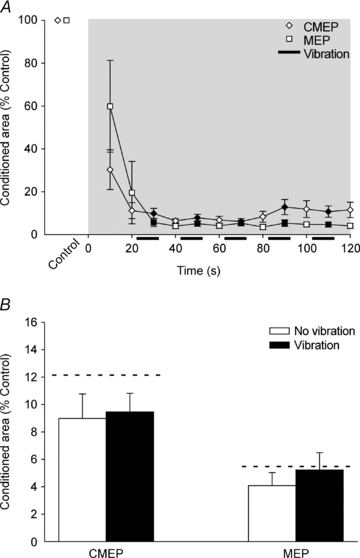
A, data are mean values (± SEM) for conditioned CMEPs and MEPs. The shaded box indicates the sustained MVC and filled symbols indicate data points collected during vibration of the distal biceps tendon. Conditioned area, expressed as a percentage of the control area without vibration, decreased rapidly for both CMEPs and MEPs. B, mean area (± SEM) of conditioned CMEPs and MEPs recorded with (30, 50, 70, 90 and 110 s) and without (40, 60, 80, 100 and 120 s) vibration during the 2 min MVC. Dashed horizontal lines represent the mean value of conditioned potentials recorded at similar time points in our earlier study without vibration (McNeil et al. 2009). Vibration did not significantly increase the size of conditioned CMEPs (P = 0.766) or MEPs (P = 0.075). Conditioned MEPs tended to be smaller than conditioned CMEPs but the difference was not statistically significant (P = 0.080).
Discussion
We previously speculated that the dramatic reduction of CMEPs (and MEPs) in the silent period during a maximal effort was caused by decreased motoneurone excitability to descending input through altered motoneurone properties and disfacilitation due to decreased muscle spindle discharge (McNeil et al. 2009). However, tendon vibration during a sustained maximal effort did not affect conditioned CMEP size. This makes it unlikely that motoneurone disfacilitation due to decreased muscle spindle discharge contributed significantly to the fatigue-related reduction of motoneurone excitability during the silent period. Therefore, we argue below that a change to intrinsic motoneurone properties caused by repetitive discharge is the predominant mechanism.
Muscle spindles deliver excitatory input to motoneurones (see Introduction) and therefore a reduction in muscle spindle discharge will lower the net excitability of motoneurones. Macefield and colleagues (1991) demonstrated rapid and marked reductions in muscle spindle discharge rates during sustained weak contractions (<30% MVC). The decline in discharge rate was positively correlated to contraction strength leading the authors to suggest that a greater reduction of spindle discharge rate was possible during maximal contractions. The conditioned CMEPs are recorded during the silent period and it has been shown that muscle relaxation gets progressively slower during the silent period in a 2 min MVC (Todd et al. 2005, 2007; Fig. 1). Prior to conducting this experiment, we believed that the slowed muscle relaxation would reduce stretch-induced muscle spindle discharge and therefore cause a progressive disfacilitation of the motoneurones in the silent period (McNeil et al. 2009). Indirect support for fatigue-related withdrawal of muscle spindle input may be provided by the disappearance of small bursts of EMG within the silent period (Taylor et al. 1996; Fig. 1). This activity has been proposed to reflect long-latency responses to the TMS which may include muscle spindle input to motoneurones (Holmgren et al. 1990). Despite these various lines of evidence, the use of tendon vibration to increase excitatory muscle spindle input to the motoneurones had a negligible impact on conditioned CMEP size in a 2 min MVC (Fig. 5).
Given this somewhat surprising result, and the absence of an effect of vibration on conditioned potentials during control MVCs, one might question the efficacy of the tendon vibration used in this study. However, we are confident that the vibration excited muscle spindles because we used the optimal frequency range (80–100 Hz) as determined by Roll and colleagues (1989), and the vibration elicited illusory movements and tonic vibration reflexes during relaxation and an increase in torque during sustained MVCs (Fig. 2). Given the indirect evidence that vibration caused an increase in excitation to motoneurones during fatiguing maximal efforts (see also Bongiovanni & Hagbarth, 1990), the failure of vibration to increase the conditioned CMEP can be interpreted in two ways. First, the slowing of muscle relaxation with fatigue and consequent reduction in muscle spindle firing during the silent period is of little importance to the reduction of the CMEP. Second, the CMEP (and MEP) is not sensitive to the facilitatory effects of muscle spindle input to the motoneurones. If the CMEP is not sensitive to the effects of muscle spindle input then the withdrawal of spindle input with fatigue should not affect the CMEP. In either scenario, the data do not favour the withdrawal of spindle input to motoneurones as the principal mechanism for the rapid reduction of the CMEP during a sustained maximal effort.
The present data show that the CMEP is depressed during a sustained maximal effort and that this does not depend on altered descending drive (as this is eliminated in the silent period) and is minimally dependent on decreased muscle spindle input. The profound depression of the CMEP suggests a decrease in motoneurone excitability. The CMEP (and MEP) evoked in biceps has a strong monosynaptic contribution from corticospinal neurones (e.g. Petersen et al. 2002), although it is likely to also have a non-monosynaptic component which will contribute to the size of the response. This means that pre-motoneuronal mechanisms may contribute to the reduction in the CMEP, but the near-abolition of the response with fatigue is unlikely without a decrease in the excitability of the motoneurones themselves. In the past, the firing of fatigue-sensitive small-diameter muscle afferents has been postulated to cause reflex inhibition of the motoneurones (Bigland-Ritchie et al. 1986; Garland, 1991; Sacco et al. 1997). However, we have demonstrated that firing of groups III and IV muscle afferents, maintained by ischaemia after a 2 min MVC, does not depress CMEPs in biceps at rest or during maximal contractions (Taylor et al. 2000; Butler et al. 2003). Indeed, these afferents tend to excite the elbow flexor motoneurone pool (Martin et al. 2006, 2008). Together these findings lead us to conclude that changes to intrinsic motoneurone properties are the principal cause for the fatigue-related suppression of the conditioned CMEP. However, this conclusion is based on exclusion and we have not identified the exact mechanism.
In addition to inducing changes at a motoneuronal level, vibration has been shown to augment MEPs at a motor cortical level (Kossev et al. 1999). MEPs are also altered by the physiological changes in muscle spindle firing produced through manipulation of the thixotropic properties of intrafusal muscle fibres (Stuart et al. 2002). For these reasons, we tested the effect of vibration on conditioned MEPs in the silent period. In a relaxed muscle, Rosenkranz & Rothwell (2003) reported that MEPs conditioned by a preceding suprathreshold stimulus were decreased with vibration when expressed as a percentage of unconditioned MEPs. However, as data on absolute conditioned MEP sizes were not presented, it is unclear if vibration decreased conditioned MEP size or caused an increase which was smaller in magnitude than that seen for unconditioned MEPs. Our results show that tendon vibration had a negligible effect on the size of the conditioned MEP in the silent period before fatigue or on the additional fatigue-related suppression of conditioned MEPs during a 2 min MVC (Fig. 5). Although not statistically significant, conditioned MEPs tended to be suppressed to a greater extent than CMEPs during the last 90 s of contraction (Fig. 5) which is similar to our previous result (McNeil et al. 2009). Thus, while we maintain that the majority of suppression of the conditioned MEP occurs at a spinal level, an additional intracortical component cannot be completely ruled out.
This study was designed to address the specific question of whether reduced muscle spindle firing during the silent period after TMS is a major contributor to the fatigue-related reduction in motoneurone excitability under those conditions. We conclude that it is not, and suggest that a change in the intrinsic properties of the motoneurones makes them much less responsive to excitatory input. Indeed, one reason that changed muscle spindle firing makes little difference to the CMEP in this condition may be because the altered intrinsic properties of the motoneurones make the excitatory input from the spindles ineffective. To put our conclusions into context, when the motoneurones are tested not during the silent period but with ongoing descending drive there is a relatively small decrease in the CMEP in the final 30 s of a 2 min MVC (Butler et al. 2003; Martin et al. 2006; McNeil et al. 2009). Furthermore, we confirmed that there is an increase in muscle torque evoked by tendon vibration. Thus, it seems that sufficient ongoing physiological drive can largely overcome the reduced response of the motoneurones to synaptic input.
In conclusion, the rapid and profound suppression of the conditioned CMEP (and MEP) in the silent period which occurs during a sustained maximal effort is not ameliorated by increased muscle spindle firing and hence appears to be largely mediated by changes to intrinsic motoneuronal properties caused by repetitive activation.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia.
Glossary
Abbreviations
- CMEP
cervicomedullary motor-evoked potential
- MEP
motor-evoked potential
- MVC
maximal voluntary contraction
- TMS
transcranial magnetic stimulation
Author contributions
Each author contributed to all aspects of the study and all authors approved the final version. All experiments were performed at Neuroscience Research Australia (formerly Prince of Wales Medical Research Institute) in Sydney, Australia.
References
- Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiovanni LG, Hagbarth KE. Tonic vibration reflexes elicited during fatigue from maximal voluntary contractions in man. J Physiol. 1990;423:1–14. doi: 10.1113/jphysiol.1990.sp018007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23:10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gail P, Lance JW, Neilson PD. Differential effects on tonic and phasic reflex mechanisms produced by vibration of muscles in man. J Neurol Neurosurg Psychiatry. 1966;29:1–11. doi: 10.1136/jnnp.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol. 1999;521:749–759. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland SJ. Role of small diameter afferents in reflex inhibition during human muscle fatigue. J Physiol. 1991;435:547–558. doi: 10.1113/jphysiol.1991.sp018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Analysis of effective synaptic currents generated by homonymous Ia afferent fibers in motoneurons of the cat. J Neurophysiol. 1988;60:1946–1966. doi: 10.1152/jn.1988.60.6.1946. [DOI] [PubMed] [Google Scholar]
- Holmgren H, Larsson LE, Pedersen S. Late muscular responses to transcranial cortical stimulation in man. Electroencephalogr Clin Neurophysiol. 1990;75:161–172. doi: 10.1016/0013-4694(90)90170-o. [DOI] [PubMed] [Google Scholar]
- Jackson A, Baker SN, Fetz EE. Tests for presynaptic modulation of corticospinal terminals from peripheral afferents and pyramidal tract in the macaque. J Physiol. 2006;573:107–120. doi: 10.1113/jphysiol.2005.100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Time course and properties of late adaptation in spinal motoneurones of the cat. Exp Brain Res. 1982;46:191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. The effects of single afferent impulses on the probability of firing of external intercostal motoneurones in the cat. J Physiol. 1982;322:315–336. doi: 10.1113/jphysiol.1982.sp014039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossev A, Siggelkow S, Schubert M, Wohlfarth K, Dengler R. Muscle vibration: different effects on transcranial magnetic and electrical stimulation. Muscle Nerve. 1999;22:946–948. doi: 10.1002/(sici)1097-4598(199907)22:7<946::aid-mus22>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Macefield G, Hagbarth KE, Gorman R, Gandevia SC, Burke D. Decline in spindle support to α-motoneurones during sustained voluntary contractions. J Physiol. 1991;440:497–512. doi: 10.1113/jphysiol.1991.sp018721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macefield VG, Gandevia SC, Bigland-Ritchie B, Gorman RB, Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol. 1993;471:429–443. doi: 10.1113/jphysiol.1993.sp019908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC, Taylor JL. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol. 2009;587:5601–5612. doi: 10.1113/jphysiol.2009.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magladery JW, Porter WE, Park AM, Teasdall RD. Electrophysiological studies of nerve and reflex activity in normal man. IV. The two-neurone reflex and identification of certain action potentials from spinal roots and cord. Bull Johns Hopkins Hosp. 1951;88:499–519. [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci. 2006;26:4796–4802. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol. 2008;586:1277–1289. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Is presynaptic inhibition distributed to corticospinal fibres in man? J Physiol. 1994;477:47–58. doi: 10.1113/jphysiol.1994.sp020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Butler JE, Gandevia SC. Depression of activity in the corticospinal pathway during human motor behavior after strong voluntary contractions. J Neurosci. 2003;23:7974–7980. doi: 10.1523/JNEUROSCI.23-22-07974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Gandevia SC. The effect of electrical stimulation of the corticospinal tract on motor units of the human biceps brachii. J Physiol. 2002;544:277–284. doi: 10.1113/jphysiol.2002.024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res. 1989;76:213–222. doi: 10.1007/BF00253639. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol. 2003;551:649–660. doi: 10.1113/jphysiol.2003.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Sacco P, Newberry R, McFadden L, Brown T, McComas AJ. Depression of human electromyographic activity by fatigue of a synergistic muscle. Muscle Nerve. 1997;20:710–717. doi: 10.1002/(sici)1097-4598(199706)20:6<710::aid-mus8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Stuart M, Butler JE, Collins DF, Taylor JL, Gandevia SC. The history of contraction of the wrist flexors can change cortical excitability. J Physiol. 2002;545:731–737. doi: 10.1113/jphysiol.2002.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol. 1996;490:519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Petersen N, Butler JE, Gandevia SC. Ischaemia after exercise does not reduce responses of human motoneurones to cortical or corticospinal tract stimulation. J Physiol. 2000;525:793–801. doi: 10.1111/j.1469-7793.2000.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Butler JE, Taylor JL, Gandevia SC. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563:621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Butler JE, Martin PG, Gorman RB, Gandevia SC. Use of motor cortex stimulation to measure simultaneously the changes in dynamic muscle properties and voluntary activation in human muscles. J Appl Physiol. 2007;102:1756–1766. doi: 10.1152/japplphysiol.00962.2006. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol. 1991;29:418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]


