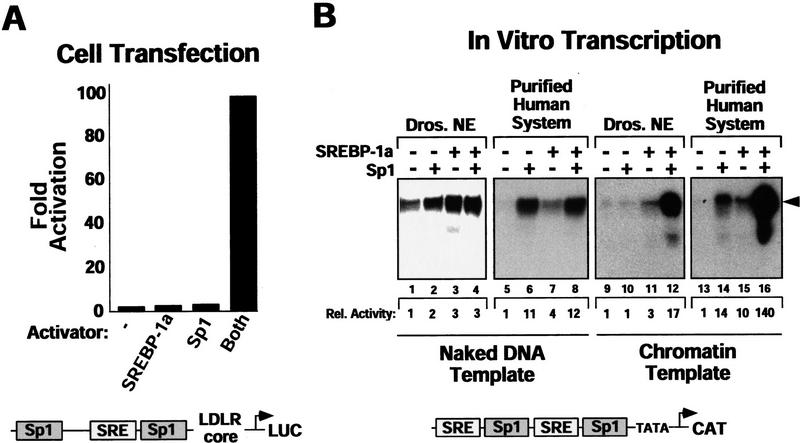
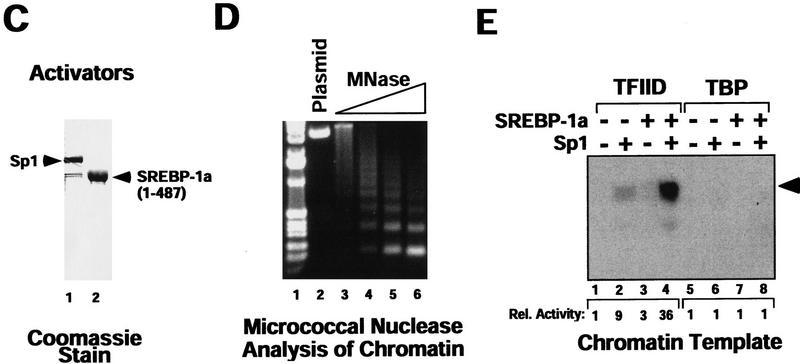
Figure 1.
Sp1/SREBP-1a-dependent transcriptional synergy in a purified in vitro transcription system requires chromatin templates and TAFs. (A) Synergistic activation of transcription by Sp1 and SREBP-1a on the LDL receptor promoter in vivo. Transient transfection of Drosophila Schneider cells with vectors directing expression of human Sp1 and SREBP (SREBP-1a amino acids 1–487) or vector alone (none) together with a reporter construct containing the human LDLR promoter and enhancer sequences fused to the luciferase reporter gene. The organization of the LDLR promoter/enhancer is schematically depicted below the bar graph. (B) Sp1 and SREBP-1a require chromatin for synergistic activation of the LDLR-derived template. PhosphorImager (Fuji) scan and autoradiographs of the in vitro transcription results with no activator added (lanes 1,5,9,13), Sp1 (lanes 2,6,10,14), SREBP-1a (lanes 3,7,11,15), or both activators added (lanes 4,8,12,16). The activity relative to basal transcription (lane 1, arbitrarily set to 1) is shown below the transcription panels (Rel. Activity). In vitro transcription reactions were assembled by purified activators, a crude Drosophila transcription system (lanes 1–4,9–12) or a highly purified human transcription system (lanes 5–8,13–16), and a LDLR promoter-derived reporter plasmid known to support SREBP-1a/Sp1 synergy in vivo. Activators were tested for activation with the crude Drosophila transcription system (lanes 9–12) or the purified human transcription system (lanes 13–16) by use of naked (lanes 1–8) or chromatin (lanes 6–16) templates. Transcription products were analyzed by primer extension and denaturing PAGE. (C) Purified activators used in transcription reactions. Human recombinant Sp1 (lane 1) was expessed by vaccinia virus infection of HeLa cells and purified from nuclear extract by wheatgerm- and DNA-affinity chromatography. Human His-tagged SREBP-1a (amino acids 1–487, corresponding to the nuclear proteolytic product) (lane 2) was generated by baculovirus infection of Sf9 insect cells and purified over Ni-NTA and DNA affinity columns. (D) Analysis of the LDLR chromatin template by micrococcal nuclease digestion. The DNA template was assembled into chromatin by use of a Drosophila chromatin assembly system and the resulting nucleosomal template was analyzed by digestion with increasing concentrations of micrococcal nuclease. (E) Activation by Sp1/SREBP-1a on the LDLR chromatin template requires TAFs. This panel shows the results of in vitro transcription reactions by use of the purified transcription system and chromatin templates containing either no activators (lanes 1,5), Sp1 (lanes 2,6), SREBP-1a (lanes 3,7), or both activators (lanes 4,8) in the presence of either immuno-purified human TFIID (lanes 1–4) or recombinant human TBP (lanes 5–8).