Figure 2.
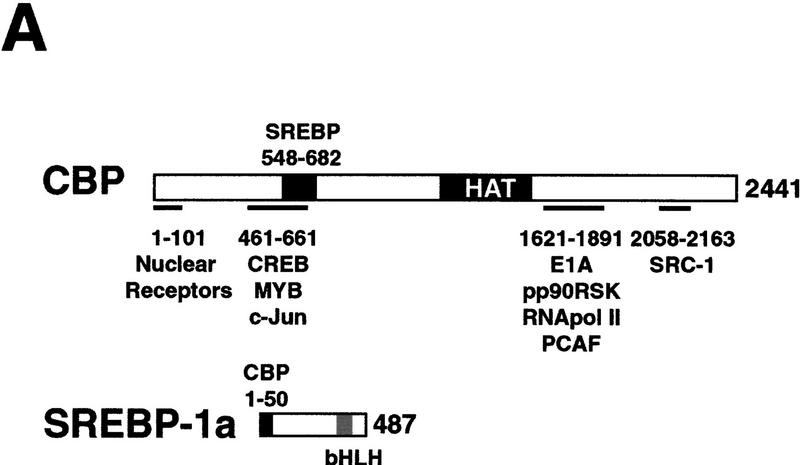
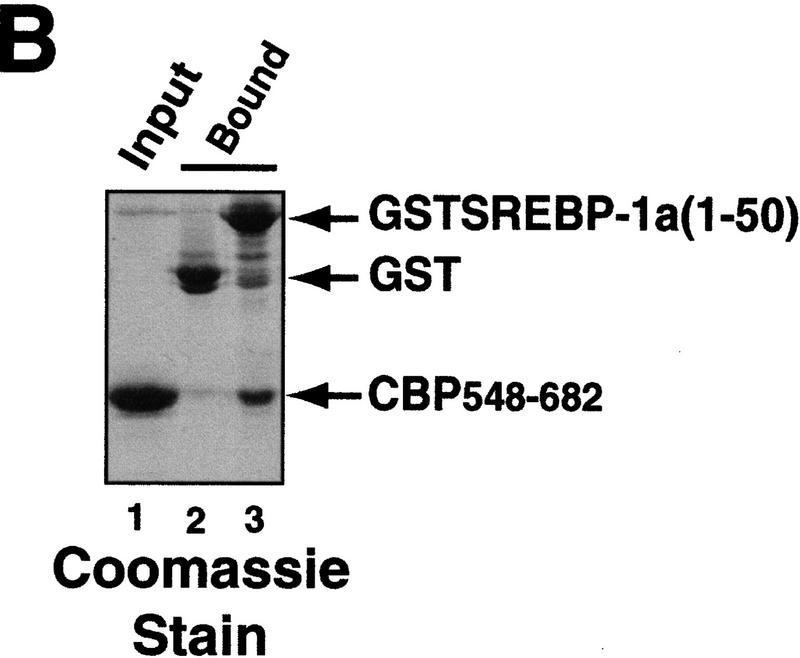
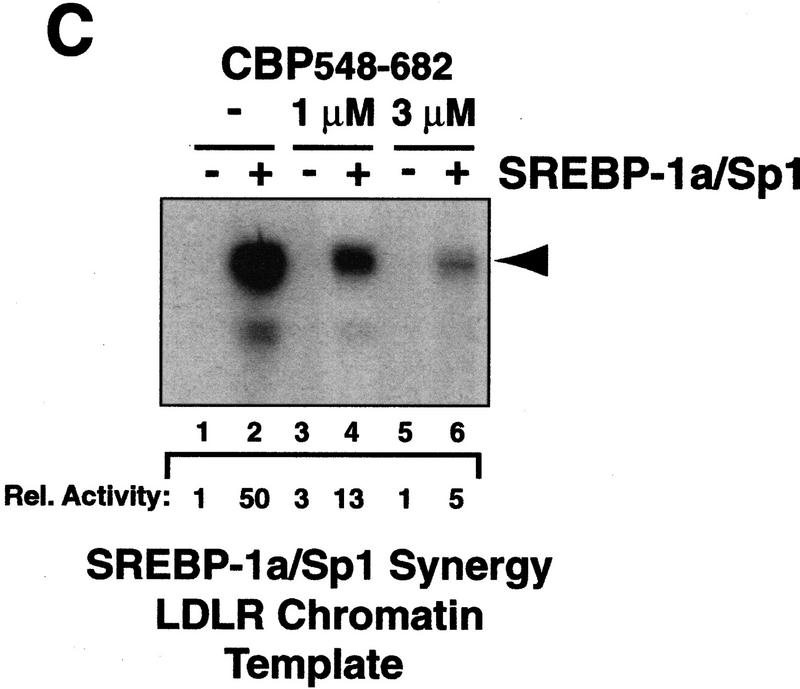
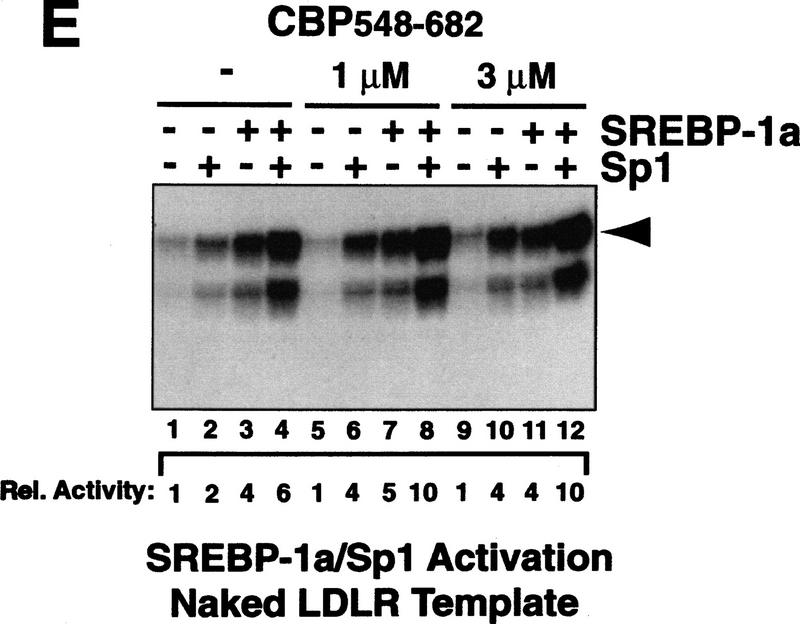
Interaction between CBP and SREBP-1a is implicated in chromatin-specific transcriptional synergy. (A) Schematic depiction of the domains of CBP that are involved in protein–protein interactions and HAT activity. The CBP domain interacting with the amino-terminal 50 amino acids of SREBP-1a was determined by partial V8 protease digestion. The regions that are involved in interaction between CBP and SREBP-1a are highlighted by solid boxes in both proteins. (B) Binding assay with CBP548–682 and GST–SREBP-1a (amino acids 1–50). Purified CBP548–682 was incubated with glutathione-agarose beads prebound to either GST (lane 2) or GST–SREBP-1a (amino acids 1–50) (lane 3). Lane 1 shows the input CBP548–682 (50%). The binding of CBP548–682 to the beads was analyzed by SDS-PAGE and visualized by Coomassie staining. (C) Effect of adding CBP548–682 protein (lanes 3–6) to a highly purified transcription system using the LDLR-promoter chromatin template and SREBP-1a/Sp1. (D) Effect of adding CBP548–682 protein (lanes 3–6) to a highly purified transcription system using a HIV–LTR chromatin template and NF-κB/Sp1. (E) The same CBP548–682 protein fragment was added (lanes 5–12) to a highly purified transcription system using a naked LDLR-promoter template and SREBP-1a/Sp1.