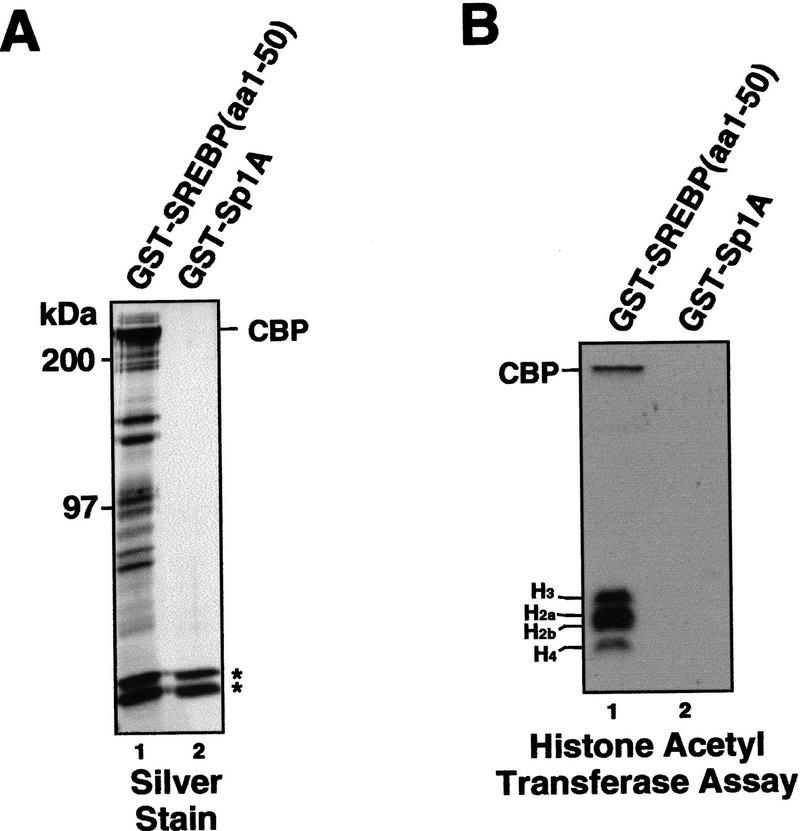
Figure 4.
A CBP-containing multiprotein coactivator associates selectively with the activation domain of SREBP-1a and mediates HAT activity. (A) A series of proteins in addition to CBP associate selectively with the SREBP-1a activation domain. HeLa cell nuclear extract was incubated with affinity resins containing the activation domains of SREBP-1a (GST–SREBP-1a amino acids 1–50) (lane 1) or Sp1 (GST–Sp1A amino acids 83–262) (lane 2) and after extensive washing, the bound fractions were analyzed by SDS-PAGE followed by silver staining (shown) and immunoblotting. The migration of CBP is indicated. The two asterisks mark the positions of nonspecific proteins binding to the GST portion of the fusion proteins. (B) The SREBP activation domain recruits HAT activity. Pulldowns from HeLa nuclear extract with GST–SREBP (amino acids 1–50) (lane 1) and GST–Sp1A (amino acids 83–262) (lane 2) were tested for HAT activity by incubating the washed beads with purified Drosophila core histones, 3H-labeled acetyl CoA, and Na Butyrate. The reactions were analyzed by separation on a 15% SDS polyacrylamide gel followed by autoradiography. The migration of core histones and CBP is indicated at left