Abstract
There has been an increased influx of probiotic products in the Indian market during the last decade. However, there has been no systematic approach for evaluation of probiotics in food to ensure their safety and efficacy. An initiative was, therefore, taken by the Indian Council of Medical Research (ICMR) along with the Department of Biotechnology (DBT) to formulate guidelines for regulation of probiotic products in the country. These guidelines define a set of parameters required for a product/strain to be termed as ‘probiotic’. These include identification of the strain, in vitro screening for probiotic characteristics, animal studies to establish safety and in vivo animal and human studies to establish efficacy. The guidelines also include requirements for labeling of the probiotic products with strain specification, viable numbers at the end of shelf life, storage conditions, etc., which would be helpful to the consumers to safeguard their own interest.
Keywords: Efficacy, guidelines, probiotics, safety
Introduction
Probiotics are live microorganisms that have a beneficial effect on human health. The concept of probiotics was introduced in early 20th century by Elie Metschnikoff1, it however, gained momentum in recent past with considerable growth in functional food market. India is fast emerging as a potential market for probiotics in food with the major players/suppliers being Nestle, Mother Dairy, Danisco, Chr Hansen, Yakult and Danone. According to a recent market research report, ‘Probiotics Market (2009-2014)’ the global probiotics market generated US $15.9 billion in 2008 and is expected to be worth US $ 32.6 billion by 2014 with a compound annual growth rate of 12.6 percent from 2009 to 20142. The probiotic product industry in India is an estimated ![]() 20.6 million with a projected annual growth rate of 22.6 per cent until 20153.
20.6 million with a projected annual growth rate of 22.6 per cent until 20153.
There are no regulatory guidelines for probiotic foods in India. Hence, there would always be a possibility of spurious and ineffective products with false claims being marketed. Hence, it becomes imperative that these products are standardized to fulfill some essential prerequisite conditions before being labelled as a ‘probiotic product’.
A Task Force was, therefore, constituted by the ICMR, comprising experts from varied fields to draft guidelines for evaluation of probiotics in India. The Task Force took into consideration the guidelines available in different parts of the world4–10 and deliberated on the various aspects to be covered11–15. The following guidelines have been drafted for scientific purpose for evaluating probiotics foods. The guidelines hereafter will be known as “ICMR-DBT guidelines”.
ICMR-DBT Guidelines for Evaluation of Probiotics in Food
-
Scope: The guidelines deal with the use of probiotics in food and provide requirements for assessment of safety and efficacy of the probiotic strain and health claims and labeling of products with probiotics.
Note: These guidelines are not meant for probiotics which by definition would come under drugs, beneficial microorganisms not used in foods or genetically modified microorganisms (GMOs).
Definition of probiotics: Probiotics are ‘live microorganisms which when administered in adequate amounts confer a health benefit on the host’ (FAO/WHO, 2002)4.
-
Genus, species and strain identification: Effects of probiotics are strain specific. Strain identity is important to link a strain to a specific health effect as well as to enable accurate surveillance and epidemiological studies. Both phenotypic and genotypic tests should be done using validated standard methodology. Nomenclature of the bacteria must conform to the current, scientifically recognized names as per the International Committee on Systematics of Prokaryotes (ICPS)16. The Figure indicates various steps for evaluation of candidate probiotic strains.
The current molecular techniques used for identification include PCR based techniques, 16S rRNA sequencing and DNA fingerprinting techniques like ribotyping and pulsed field gel electrophoresis (PFGE).
It is recommended that probiotic strains in use in India should be deposited in an internationally recognized culture collection/repository.
-
In vitro tests to screen potential probiotic strains: The following in vitro tests * with standard methodology are recommended for screening putative probiotic strains:
-
(i)Resistance to gastric acidity,
-
(ii)Bile acid resistance,
-
(iii)Antimicrobial activity against potentially pathogenic bacteria (acid and bacteriocin production),
-
(iv)Ability to reduce pathogen adhesion to surfaces,
-
(v)Bile salt hydrolase activity.
These tests are based on the hostile gut environment which these mimic under in vitro conditions. The cultures evaluated as probiotics based on these tests should be subjected to preclinical validation in appropriate animal models before clinical trials are conducted in human subjects.
-
(i)
In vivo safety studies in animal models: Assessment of the acute, subacute and chronic toxicity of ingestion of extremely large amounts of probiotics should be carried out for all potential strains. Such assessment may not be necessary for strains with established documented use.
In vivo efficacy studies in animal models: To substantiate in vitro effects, appropriate, validated animal models must be used first, prior to human trials.
-
Evaluation of safety of probiotics for human use: In recognition of the importance of assuring safety, even among group of bacteria that are Generally Recognized as Safe (GRAS) **, probiotics strains needs to be characterized at a minimum with the following tests:
-
(i)Determination of antibiotic resistance patterns. It should be ascertained that any given probiotic strain is not at significant risk with regard to transferable antibiotic resistance.
-
(ii)Assessment of undesirable side-effects.
-
(iii)If the strain under evaluation belongs to a species that is a known mammalian toxin producer or of haemolytic potential, it must be tested for toxin production and haemolytic activity respectively.
Assessment of lack of infectivity by a probiotic strain in immunocompromised individuals would be an added measure.
-
(i)
-
Evaluation of efficacy studies in humans: The principal outcome of efficacy studies on probiotics should be proven with similar benefits in human trials, such as statistically and clinically significant improvement in condition, symptoms, signs, well-being or quality of life, reduced risk of disease or longer time to next occurrence or faster recovery from illness. Each of the parameter should have proven correlation with the probiotics tested.
Probiotics delivered in food may not be tested in Phase 3 studies (effectiveness), unless the product makes a specific health claim wherein it becomes imperative to generate the required evidence necessitating carrying out Phase 3 studies.
If a probiotic food has a record of documented long and safe use outside the country, the data regarding this could be reviewed and deemed as sufficient to allow its marketing within the country. However, labeling of health benefits may require evaluation in a different manner. While taking into account studies done abroad, efficacy studies of probiotics (which are of proven benefit in ‘other’ populations) should also be conducted on Indian subjects. It is recommended that such ‘bridging’ human trials should comply with the principles laid down by the Drug Regulatory Authority. Adverse effects, if any, should be monitored and incidents reported to the appropriate authority.
Effective dosage of probiotic strain/strains: The minimal effective dose or the level of viable cells of the probiotic strain in terms of cfu/ml/day in the carrier food that demonstrates general health promoting functions or well being or specific health claims in target population should be clearly indicated.
-
Labeling requirements: In addition to the general labeling requirements under the food laws, the following information should also be mentioned on the label18,19.
-
(i)Genus, species and strain designation following the standard international nomenclature.
-
(ii)The minimum viable numbers of each probiotic strain should be specified at the level at which efficacy is claimed and at the end of shelf- life.
-
(iii)Evidence-based health claim(s) should be clearly stated.
-
(iv)The suggested serving size to deliver the minimum effective quantity of the probiotic related to the health claim.
-
(v)Proper storage conditions to be mentioned.
-
(i)
Manufacturing and handling procedures: Adequate quality assurance programmes should be in place. Good Manufacturing Practices should be followed in the manufacture of probiotic foods. The Codex General Principles of Food Hygiene and Guidelines for Application of Hazard Analysis and Critical Control Point (HACCP)20 should be followed.
Fig.
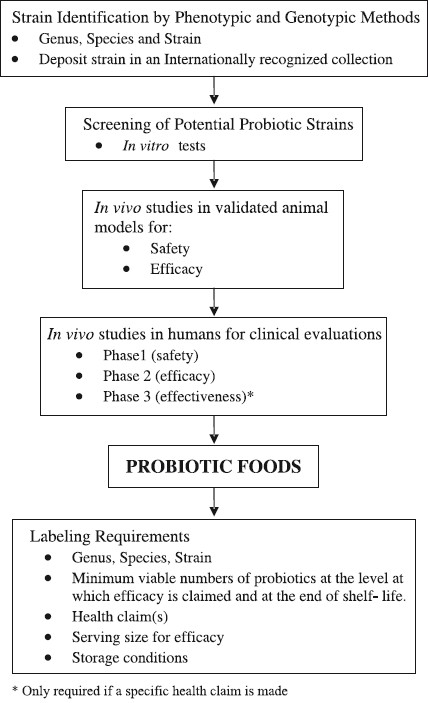
Guidelines for evaluation of candidate probiotic strains.
References
- 1.Metchnikoff E. The prolongation of life: Optimistic studies. London: W. Heinemann; 1907. Lactic acid as inhibiting intestinal putrefaction; pp. 161–83. [Google Scholar]
- 2.Probiotic Market- Advanced Technologies and Global Market (2009 - 2014). By: marketsandmarkets.com. Publishing Date: September 2009. Report Code: FB 1046. [accessed on February 15, 2011]. Available from http://www.marketsandmarkets.com/Market-Reports/probiotic-market-advanced-technologies-and-global-market-69.html .
- 3.Probiotics in Foods and Beverages-Strategic Assessment of the Indian Market. Frost & Sullivan (2009) 2009. Dec 31, [accessed on February 15, 2011]. Available from: http://www.marketsandmarkets.com/Market-Reports/probiotic-market-advanced-technologiesand-global-market-69.html .
- 4.Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. London, Ontario, Canada: 2002. April, May. Guidelines for evaluation of probiotics in food. [Google Scholar]
- 5.EFFCA Guidelines for Probiotics in Food and Dietary Supplements. European Food and Feed Culture Association. 2008. [accessed on January 15, 2009]. Available from: http://bbs.bio668.com/simple/index.php?t35919.html .
- 6.Establishing standards for Probiotics: ISAPP's Role. International Scientific Association for Probiotics and Prebiotics. 2005. [accessed on October 10, 2008]. Available from: http://www.isapp.net/docs/probiotic%20standards%20justification.pdf .
- 7.Evidence for safety and effi cacy of fi nished natural health products, Natural Health Products Directorate, Canada. 2006. Dec, [accessed on October 9, 2008]. Available from: http://dsp-psd.pwgsc.gc.ca/collection_2007/hc-sc/H164-39-2006E.pdf .
- 8.IPA releases Guidelines for probiotic supplements. International Probiotics Association. 2008. [accessed on November 15, 2008]. Available from: http://www.naturalproductsinsider.com/hotnews/ipa-guidelinesprobiotic-supplements.html .
- 9.Opinion of the Scientifi c Committee on a request from EFSA on the introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA J. 2007;587:1–16. [Google Scholar]
- 10.Washington DC: U.S. Department of Health and Human Services. Food and Drug Administration; 2006. Dec, Guidance for Industry on Complementary and Alternative Medicine Products and their Regulation by the Food and Drug Administration. Draft Guidance. [Google Scholar]
- 11.Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Córdoba, Argentina: 2001. Oct 1-4, Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. [Google Scholar]
- 12.Von Wright A. Regulating the safety of probiotics – The European Approach. Curr Pharm Des. 2005;11:17–23. doi: 10.2174/1381612053382322. [DOI] [PubMed] [Google Scholar]
- 13.Przyrembel H. Consideration of possible legislation within existing regulatory frameworks. Am J Clin Nutr. 2001;73:S471–5. doi: 10.1093/ajcn/73.2.471s. [DOI] [PubMed] [Google Scholar]
- 14.Reid G. The importance of guidelines in the development and application of probiotics. Curr Pharm Des. 2005;11:11–6. doi: 10.2174/1381612053382395. [DOI] [PubMed] [Google Scholar]
- 15.Specifications for the Identity and Purity of Food Additives and their Toxicological Evaluation: Some Antimicrobials, Antioxidants, Emulsifiers, Stabilizers, Flour-Treatment Agents, Acids and Bases. Ninth Report, FAO Nutrition Meetings Report Series, 1966, No. 40. World Health Organization Techn. Rep Ser No. 339. 1966 [PubMed] [Google Scholar]
- 16.International Committee on Systematics of Prokaryotes (ICPS) [accessed on November 14, 2010]. Available from: http://www.the-icsp.org/
- 17.US Food and Drug Administration. Generally Recognized As Safe (GRAS) [accessed on June 29, 2011]. Available from: http://www.fda.gov/Food/FoodIngredientsPackaging/GenerallyRecognizedasSafeGRAS/default.htm .
- 18.Reid G. Cordoba, Argentina: 2001. Oct 1-4, Regulatory and clinical aspects of dairy probiotics. Background paper for FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food including Powder Milk and Live Lactic Acid Bacteria. [Google Scholar]
- 19.Saldanha LG. US Food and Drug Administration regulations governing label claims for food products, including probiotics. Clin Infect Dis. 2008;46(Suppl 2):S119–21. doi: 10.1086/523328. [DOI] [PubMed] [Google Scholar]
- 20.Codex Alimentarius Commission. Recommended International Code of Practice: General Principles of Food Hygiene. CAC/RCP 1-1969, Rev 4-2003. [accessed on June 29, 2011]. Available from: www.codexalimentarius.net/download/standards/23/cxp_001e.pdf .


