Abstract
Up to 30% of hospitalized critically ill patients may have a rise in serum creatinine concentration. In addition to history and physical examination, there is diagnostic value in assessing urinary electrolytes, solute excretion, and urine flow in these patients. The correct interpretation of these urinary parameters can avoid unnecessary volume overload and mechanical ventilation, risk factors for increased mortality in patients with rising serum creatinine. The present article also discusses the role of arterial underfilling in causing prerenal azotemia in the presence of an increase in total body sodium and extracellular fluid expansion. As with extracellular fluid volume depletion, arterial underfilling secondary to impaired cardiac function or primary arterial vasodilation can delay or prevent recovery from ischemic or toxic acute tubular necrosis. The present brief review discusses the various aspects of the correct interpretation of urinary electrolytes, solute excretion, and urine flow in the setting of a rising serum creatinine concentration.
Sodium and its anion constitute the primary extracellular solutes determining tonicity.1 Thus, sodium is the major determinant of extracellular fluid volume (ECFV). In normal humans, the kidney is primarily responsible for maintaining the homeostasis of total body sodium. With a sodium-restricted diet (40 to 50 mEq/d), urinary sodium concentration decreases to less than 10 mEq/L within three to five days. During this time period, a negative sodium balance approximating 1.5 L of saline occurs in individuals previously on normal sodium diets (150 to 200 mEq/d). Whereas the kidney senses the resultant decrease in total body sodium during a sodium-restricted diet, and thereby conserves sodium, the most astute clinician would not be able to detect any change in sodium content on physical examination. The normal kidney, therefore, is much better at detecting modest changes in total body sodium and, thus, ECFV.
In normal humans, this exquisite modulation of total body sodium by the kidney involves both acute and chronic mechanisms.1 Sodium restriction is associated with stimulation of the renin-angiotensin-aldosterone system. Angiotensin II then stimulates the sympathetic nervous system. Both angiotensin and α-adrenergic stimulation increases tubular sodium reabsorption very rapidly. However, the sodium-retaining effect of aldosterone at the collecting duct takes longer because of the time necessary for new protein synthesis involving the epithelial sodium channel, the Na/K-ATPase, and the ROMK potassium channel. These effects of aldosterone are best determined by a decrease in urinary sodium concentration and an increase in urinary potassium concentration.
While these urinary electrolytes in normal subjects provide a sensitive index of total body sodium, plasma sodium concentration does not. A low plasma sodium concentration can be associated with an increase in total body sodium (heart failure or cirrhosis; hypervolemic hyponatremia), a decrease in total body sodium (diuretic use, primary adrenal insufficiency, or gastrointestinal losses; hypovolemic hyponatremia), or a near-normal total body sodium (syndrome of antidiuretic hormone secretion [SIADH]; euvolemic hyponatremia). However, assessment of urinary sodium concentration and, particularly, fractional excretion of sodium (FENa) in these hyponatremic disorders is of diagnostic value where there is oliguria.2
CIRCUMSTANCES WHERE URINARY SODIUM CONCENTRATION DOES NOT REFLECT TOTAL BODY SODIUM
The presence of diuretics negates the value of urinary sodium concentration as an index of total body sodium. When the assessment of ECFV is pivotal for clinical care, the diuretics may be temporarily discontinued (for example, Lasix: last 6 h) and the urinary electrolytes reassessed after the duration of action of the diuretics is passed; 24 to 48 h is generally sufficient. There are other circumstances when the normal kidney does not maximally conserve sodium in spite of a decrease in ECFV. Bicarbonaturia, as may occur with metabolic alkalosis or proximal tubular acidosis (Fanconi's syndrome), may not be associated with minimal urinary sodium concentration in the presence of decreased ECFV. The intraluminal negativity caused by bicarbonate can decrease sodium reabsorption, thereby increasing urinary sodium concentration in spite of a decreased ECFV. In this setting of decreased ECFV, urinary chloride concentration will be very low and, therefore, of clinical value.3 Other causes of an increase in solute excretion may also increase urinary sodium losses by the normal kidney in spite of a decrease in total body sodium and decreased ECFV. These include the diabetic patient with glucosuria or the patient who has received mannitol. The increase in urinary solute excretion decreases tubular sodium reabsorption, thereby increasing urinary sodium excretion in spite of a decrease in total body sodium.
Of course in patients with established acute tubular necrosis (ATN) secondary to ischemia, toxins, or both, tubular function is impaired and urinary sodium concentration will not be minimal, even with substantial ECFV depletion. With chronic kidney disease (glomerular filtration rate [GFR] less than 60 ml/min), the renal response to dietary sodium restriction is delayed and not maximal, but over several days, urinary sodium concentration can still decrease in patients with chronic kidney disease who are not at end-stage.4
There are rare circumstances where the clinical course is that of ATN, but the urinary sodium concentration is low, at least initially. Burn patients receiving several liters of glucose and water daily for insensible losses can also have urinary sodium concentrations less than 20 mEq/L in spite of nonoliguric ATN. Early in sepsis, the urinary sodium concentration may be less than 20 mEq/L, but then, without effective intervention, may progress to ATN with high urinary sodium concentrations.5 Early in contrast-mediated acute renal dysfunction, there is renal vasoconstriction with intact tubular function.6 Thus, an increase in serum creatinine may occur with a urinary sodium concentration less than 20 mEq/L. These patients, however, may also progress to ATN with an elevation of urinary sodium concentration.
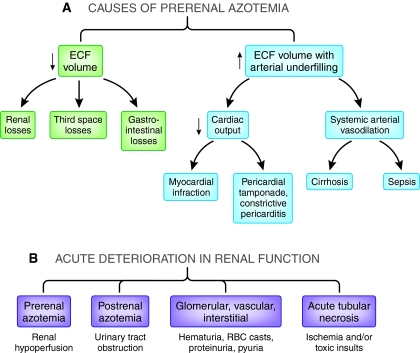
Assuming the kidney only conserves sodium with ECFV depletion may also be misleading. The normal kidney can conserve sodium, as demonstrated by a low urinary sodium concentration, in the presence of an expanded ECFV with arterial underfilling. The predominant stimulus of the normal kidney to retain sodium is not ECFV depletion or even decreased total plasma volume. Even with expanded ECFV, the arterial baroreceptors in the carotid sinus, aortic arch, and juxtaglomerular apparatus are unloaded with reversal of tonic inhibition to central nervous system, either by a decrease in stroke volume (low cardiac output failure) or primary systemic arterial vasodilation (cirrhosis). Since the arterial circulation constitutes approximately 15% of total circulation, an increased total plasma volume on the venous side of the circulation (average, 85% of total) can be associated with ECFV expansion, yet arterial underfilling and renal sodium retention occurs (Figure 1A).7
Figure 1.
(A) With prerenal azotemia, tubular function is intact and is responding to renal hypoperfusion. (B) Acute Kidney Injury (AKI) has recently been suggested to replace acute renal failure (ARF), because kidney was preferred to renal and injury was preferred to failure. I have used acute tubular necrosis (ATN) and prerenal azotemia because that is the language of the literature cited here. In either case, the systematic diagnostic approach of acute renal dysfunction must include these four entities.
FRACTIONAL EXCRETION OF SODIUM (FENA) AND FRACTIONAL EXCRETION OF UREA (FEUREA) WITH PRERENAL AZOTEMIA
A prospective study examined the validity of fractional excretion of sodium (FENa) to distinguish prerenal azotemia from established ATN.8 This is important, since one of the most common causes of ATN may be prolonged prerenal azotemia. FENa is, in general, more sensitive than urinary sodium concentration because plasma sodium concentration is factored into the calculation of FENa. Thus, FENa becomes particularly useful when plasma sodium concentration is abnormal. In this study, the baseline serum creatinine was within the normal range (< 1.4 mg/dl), and diuretic use, glucosuria, and mannitol or bicarbonaturia were not present.8 The FENa was less than 1.0 in 85 to 94% of those patients who had prerenal azotemia, as documented by reversal of kidney function secondary to interventions such as fluid resuscitation or improved cardiac output within 24 to 72 h. In those patients who did not reverse their elevated plasma creatinine, and thus had oliguric ATN, the FENa was less than 1.0 in only 0 to 4% of patients.
Some studies that have attempted to correlate FENa with the clinical course of acute renal dysfunction have not controlled factors that can make urinary results difficult to interpret including diuretic use, glucosuria, or advanced chronic kidney disease. Even with careful control of these factors, there will be approximately 20% of patients who do not clearly fall into either the prerenal or ATN group; these may be the patients who are progressing from prerenal azotemia to ATN. Thus, following urinary sodium concentration and FENa in a given patient may be helpful.
A paper was also published several years ago indicating that fractional excretion of urea (FEurea) may be more helpful than FENa in distinguishing prerenal azotemia from ATN in patients on diuretics.9 The hypothesis for this notion is as follows: Since urea reabsorption in prerenal states—such as ECFV depletion, heart failure, or cirrhosis—is enhanced in the proximal tubule before the sites of diuretic action in the downstream tubule, particularly the NKCC2 cotransporters in the loop of Henle and the NCCT cotransporters in the distal convoluted tubule, the FEurea should be lower compared with ATN, which involves impaired function in the proximal tubule as well. In contrast, determination of the ultimate urinary sodium concentration and FENa occurs after the tubular site of action of diuretics, and thus cannot distinguish between prerenal and ATN when diuretics are present. Those patients with an increase in plasma creatinine who had a FEurea less than 35 exhibited a clinical course of prerenal azotemia, whereas those with FEurea greater than 35 had a clinical course compatible with ATN.9
A subsequent study attempted to validate this conclusion.10 The authors defined prerenal azotemia as transient renal dysfunction within 7 d. They assessed FENa of less than 1% and FEurea of less than 35% as an index of prerenal azotemia in patients with or without diuretics present. In patients not on diuretics, FENa had a sensitivity and specificity of 78% and 75%, respectively, as compared with a FEurea of 48% and 75%. In patients on diuretics, the sensitivity and specificity for FENa was 58% and 81%, respectively, whereas the FEurea was 79% and 33% for transient renal dysfunction. The authors concluded that, for patients not on diuretics, FENa is better than FEurea to distinguish transient prerenal azotemia versus persistent renal dysfunction from ATN. Because of low specificity, FEurea was not considered helpful in patients on diuretics.
A blood urea nitrogen (BUN) to plasma creatinine ratio >20 also favors prerenal azotemia from decreased ECFV or arterial underfilling.11 This is because urea reabsorption increases with lower urine flow rates and increased plasma arginine vasopressin, as occurs in prerenal azotemic states, whereas creatinine is filtered and secreted but not reabsorbed. However, increased catabolism, hyperalimentation, gastrointestinal hemorrhage, or corticosteroids may also increase the ratio. Moreover, low protein intake from anorexia or illness may obscure a potentially elevated ratio in patients who actually have prerenal azotemia.
ROLE OF URINARY OSMOLALITY AND FLOW RATE IN AZOTEMIC PATIENTS
Knowledge of urinary osmolality and flow rate can be helpful in caring for the patient with an increase in plasma creatinine. Oliguria is defined as a urine volume less than 400 ml/24 h when the patient is obligated to excrete 500 to 600 mOsms/d, whereas nonoliguria is higher than this volume. Patients with nonoliguric ATN may have 24-h urine volumes as high as 1 to 2 L. This is important since fluid intake in ATN patients should be urine output plus insensible loss. Thus, inadequate fluid intake in the nonoliguric patients may delay recovery from ATN. In both oliguric and nonoliguric ATN, the FENa should be substantially >2; studies in septic patients with ATN may demonstrate FENa as high as 6 to 8.12 The FENa in nonoliguric patients with ATN may, however, be less than the patient with oliguric ATN. In general, the patients with nonoliguric ATN will only need dialysis to prevent uremic symptoms approximately 35% of the time, whereas the oliguric patient generally needs dialysis in 85% of cases. Studies have shown the mortality of nonoliguric ATN is less than oliguric ATN, approximately 26% versus 50%, respectively.13
What has also been reported, but needs substantiation, is that some patients with prerenal azotemia rarely may not be oliguric.14 This is theoretically possible when maximal urinary concentration is substantially diminished, such as in the elderly or during periods of low protein intake. Another issue relative to urine volume that can be misunderstood is the patient with normal kidney function who is undergoing a large water diuresis from primary polydipsia. As urine flow increases, urinary sodium concentration decreases so that urinary sodium excretion remains constant. Therefore, a low urinary sodium concentration with polyuria (8 to 10 L/d) does not indicate ECFV depletion. With chronic urinary tract obstruction, urinary concentration is impaired; therefore, polyuria can also indicate postrenal azotemia in conditions such as benign prostatic hypertrophy.
Two recent approaches to predict prognosis in ATN are the risk, injury, failure, loss, and end-stage renal disease (RIFLE)15 and acute kidney injury network (AKIN)16 criteria. From baseline, the higher the rise in plasma creatinine at 7 d (RIFLE) or 48 h (AKIN), the worse the prognosis. These definitions have been supported in observational studies. Since 50% of patients with ATN may be nonoliguric, the urine component of these approaches is less helpful than the changes in serum creatinine.17 These approaches are useful for epidemiologic or future interventional research studies.
POTENTIAL ADVERSE CONSEQUENCES OF NOT ASSESSING URINARY ELECTROLYTES, SOLUTE, AND URINE FLOW RATE IN PATIENTS WITH A RISE IN SERUM CREATININE
When the history and physical examination do not distinguish between prerenal azotemia and ATN, fluid administration is frequently undertaken in search of reversibility. If the patient has prerenal azotemia, reversibility can occur with this approach. On the other hand, with ATN the fluid challenge, frequently several liters, may cause fluid overload, hypoxia, and the need for mechanical ventilation. Epidemiologic studies indicate that fluid overload and mechanical ventilation are associated with increased mortality in patients with ATN.18 Fluid overload in a patient with ATN can also impair cardiac function secondary to increased ventricular wall stress, functional mitral insufficiency, and endomyocardial ischemia. Such cardiac dysfunctions can delay recovery from ATN. Moreover, increased renal venous and interstitial pressure secondary to fluid overload can further decrease GFR and activate the renin-angiotensin-aldosterone system. In contrast, if the FENa is >2, in the absence of diuretics and other aforementioned caveats, the approach would be to limit fluid intake to urine output plus insensible loss rather than a fluid challenge with its attendant deleterious consequences. Whether the FEurea adds diagnostic value in patients with a rising plasma creatinine who are receiving a diuretic is controversial. The standard diagnostic approach should still include the four categories shown in Figure 1B, and evaluation of the urinalysis and urinary indices can be quite important.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Schrier RW: Renal sodium excretion, edematous disorders, and diuretic use, Philadelphia, Lippincott Williams & Wilkins, 2010 [Google Scholar]
- 2. Berl T, Schrier RW: Disorders of water homeostasis, Philadelphia, Lippincott Williams & Wilkins, 2010 [Google Scholar]
- 3. Anderson RJ, Gabow PA, Gross PA: Urinary chloride concentration in acute renal failure. Miner Electrolyte Metab 10: 92–97, 1984 [PubMed] [Google Scholar]
- 4. Schrier RW, Regal EM: Influence of aldosterone on sodium, water and potassium metabolism in chronic renal disease. Kidney Int 1: 156–168, 1972 [DOI] [PubMed] [Google Scholar]
- 5. Kikeri D, Pennell JP, Hwang KH, Jacob AI, Richman AV, Bourgoignie JJ: Endotoxemic acute renal failure in awake rats. Am J Physiol 250: F1098–F1106, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Fang LS, Sirota RA, Ebert TH, Lichtenstein NS: Low fractional excretion of sodium with contrast media-induced acute renal failure. Arch Intern Med 140: 531–533, 1980 [PubMed] [Google Scholar]
- 7. Schrier RW: Decreased effective blood volume in edematous disorders: What does this mean? J Am Soc Nephrol 18: 2028–2031, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Miller TR, Anderson RJ, Linas SL, Henrich WL, Berns AS, Gabow PA, Schrier RW: Urinary diagnostic indices in acute renal failure: A prospective study. Ann Intern Med 89: 47–50, 1978 [DOI] [PubMed] [Google Scholar]
- 9. Carvounis CP, Nisar S, Guro-Razuman S: Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int 62: 2223–2229, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Pepin MN, Bouchard J, Legault L, Ethier J: Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 50: 566–573, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Schrier RW: Blood urea nitrogen and serum creatinine: Not married in heart failure. Circ Heart Fail 1: 2–5, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Schrier RW, Wang W: Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Anderson RJ, Linas SL, Berns AS, Henrich WL, Miller TR, Gabow PA, Schrier RW: Nonoliguric acute renal failure. N Engl J Med 296: 1134–1138, 1977 [DOI] [PubMed] [Google Scholar]
- 14. Miller PD, Krebs RA, Neal BJ, McIntyre DO: Polyuric prerenal failure. Arch Intern Med 140: 907–909, 1980 [PubMed] [Google Scholar]
- 15. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P: Acute renal failure– definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waikar SS, Bonventre JV: Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20: 672–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schrier RW: Fluid administration in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 5: 733–739, 2010 [DOI] [PubMed] [Google Scholar]