Figure 2.
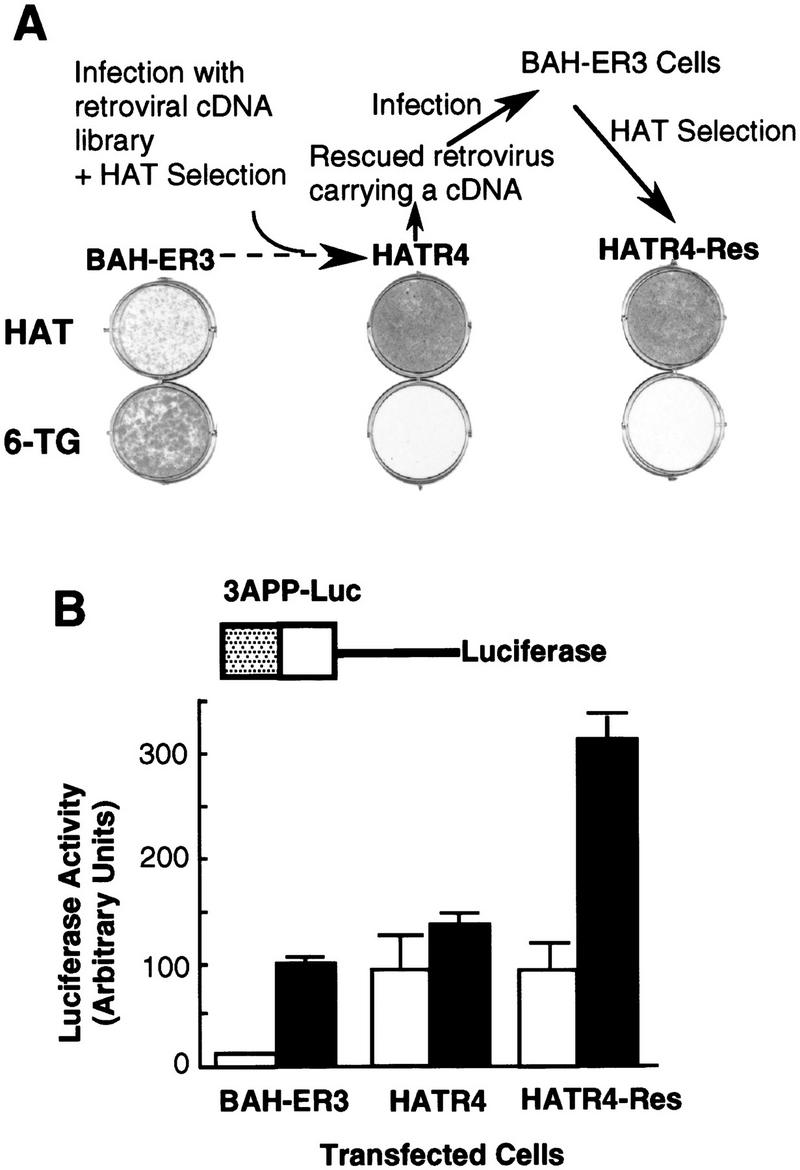
Isolation of a HAT-resistant cell clone that activates the TGF-β-inducible 3TP promoter in the absence of TGF-β. (A) After infection of BAH-ER3 cells with a retroviral cDNA library, the cells were grown in HAT medium for 2 weeks. A HAT-resistant clone, HATR4, was isolated, and then infected with wild-type Moloney retrovirus; the supernatant containing the rescued retrovirus was used to infect normal BAH-ER3 cells as described in Materials and Methods. Infected cells were also subjected to HAT selection and the resulting HAT-resistant cells, HATR4–Res cells, were isolated. BAH-ER3, HATR4, and HATR4–Res cells were plated at 5 × 104 cells/well in a 6-well plate; HAT medium or 6-TG medium was added as indicated, and cells were incubated for 10 days in the absence of TGF-β. Growing cells were stained with crystal violet. (B) On day 0, 105 cells were plated in each well of a 12-well plate. On day 2, the cells in each well were transfected with 2 μg of 3APP–Luc DNA and 0.5 μg of pSVβ. After overnight culture the cells were switched to serum-free medium with (█) or without (□) 200 pm TGF-β as indicated, and then incubated for 20 hr before being harvested for luciferase and β-galactosidase assays as described in Materials and Methods. Luciferase activities, plotted in arbitrary units, have been normalized to β-galactosidase activity. Each value represents an average of duplicate samples, and the error bar denotes the standard deviation of the duplicates.
