Figure 5.
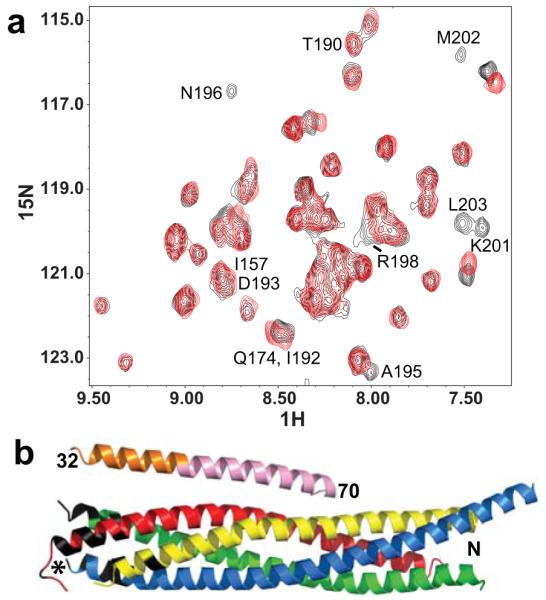
The CplxI N terminus binds to the C terminus of the SNARE complex. (a) Expansions of 1H-15N TROSY-HSQC spectra of SNARE complex with the SNAP-25 C-terminal SNARE motif 2H,15N-labeled, in the presence of CplxI 2–82 A12C-MTSL before (red contours) and after (black contours) reduction with dithionite. Selected cross-peaks are labeled with the residue number. (b) Ribbon diagram of the crystal structure of the CplxI 26–83/SNARE complex13 illustrating the PBEs caused by CplxI 2–82 A12C-MTSL on the 1H-15N TROSY-HSQC cross-peaks of the SNARE complex. CplxI is colored pink (central α-helix) and orange (accessory α-helix), Synaptobrevin-2 red, Syntaxin-1 yellow, and SNAP-25 blue and green. Residues in black correspond to well-resolved cross-peaks whose intensities decrease by more than 50% due to the PBEs induced by MTSL (residues 78, 81, 82, 86, 87 and 90 of Synaptobrevin-2, residues 242 and 247 of Syntaxin-1, and residues 73-75, 78, 80, 195, 196, 198–204 of SNAP-25). All these residues are clustered at the C terminus of the SNARE complex, and the cross-peaks from other residues in this region are not observable or overlapped. We estimate that the amide protons of these residues are approximately within 18 Å or less from the probe34. The asterisk indicates the estimated position of the MTSL probe in the bound CplxI 2–82 A12C-MTSL.
