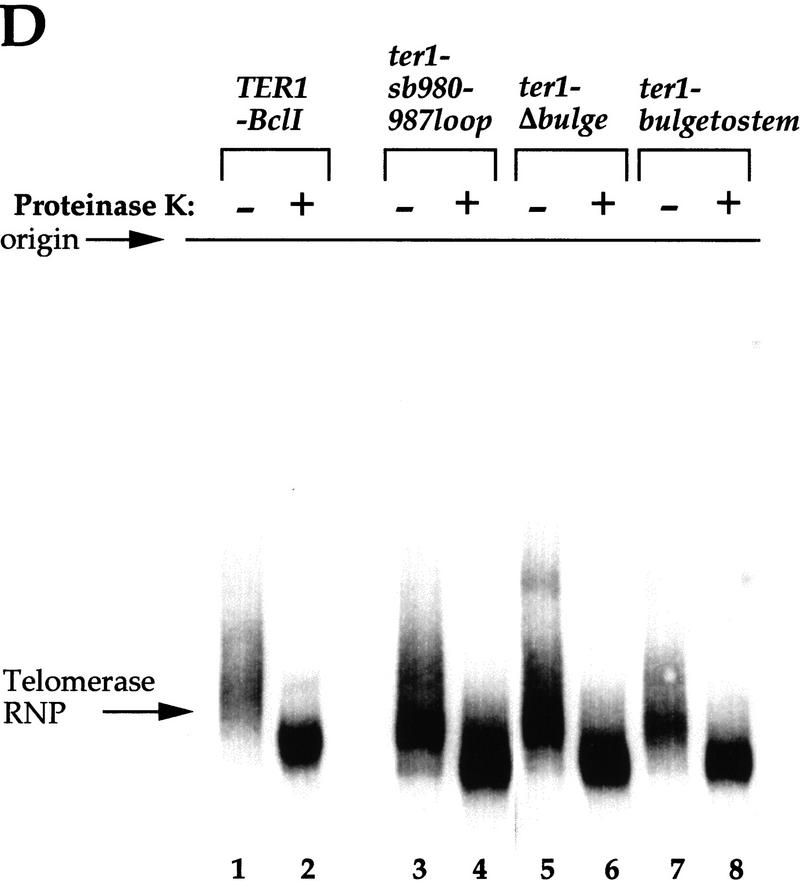
Figure 9.
(A,B) Nonfunctional TER1 mutants contain undetectable telomerase activity in vitro. DEAE fractions of extracts from strains expressing TER1 alleles were assayed for K. lactis telomerase activity in vitro with primer KL13(12). The sequence of the TER1 templating domain and the predicted alignment of the primer are shown. The boxed template residue corresponds to the site of the A → G mutation in the TER1–BclI strains examined. Terminal transferase-labeled KL13(12) primer is shown in M lanes, and the positions of the +1 products are marked correspondingly. (A) Telomerase reactions with DEAE fractionated extracts were carried out with all four dNTPs. RNase pretreatment (lanes 2,4,6) consisted of incubation of extracts with 10 μg/ml RNase A at 25°C for 5 min. Mid-template products are denoted with brackets, and near-terminal products are marked with arrowheads. A background ladder of RNase A insensitive bands was detected in lanes 5 and 6 and is most likely caused by contaminating polymerases in the fractions assayed. The asterisk marks a nontelomerase generated background band described previously (Fulton and Blackburn 1998). (B) Reactions with DEAE fractionated extracts from TER1 (lane 1), TER1–Bcl (lane 2), ter1–sb980–987loop (lane 3), ter1-Δ630–730 (lane 4), ter1-Δ20–60 (lane 5), and ter1-Δ493–580 (lane 6) strains were carried out as in A but with ddTTP substituted for dTTP. (C,D) Profiles of TER1 RNA-containing complexes in wild-type and mutant cell extracts. DEAE fractions of extracts from strains expressing TER1 alleles were fractionated on nondenaturing gels and probed with a radio-labeled TER1 DNA probe. The gel in C was run for 13 hr, whereas the gel in D was run for 10 hr.