Table 1.
Oiiistat Derivatives Prepared by the TMAL Process Followed by Either Inversion or Retention of C6 Stereochemistry during Amino Ester side Chain Introductiona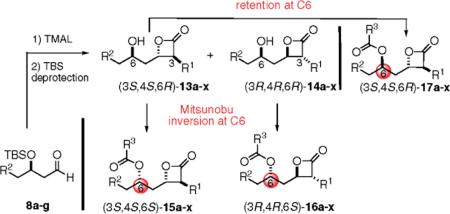
| entry | R1 | R2 | TMAL % yieldb | TMAL ratio (13:14)a | % yield Mitsunobu/ acylanon | Orlistat derivatives 15–17 |
|---|---|---|---|---|---|---|
| 1 | C6H13 | C12H25 | 58 | 13a: 14a= 6:1 | 80 (15a) |
|
| 73 (15b) | ||||||
| 80 (15c) | ||||||
| 17 (15d) | ||||||
| 17 (16a) | ||||||
|
| ||||||
| 2 | C6H13 | C10H21 | 58 | 13b: 14b = 8:1 | 87 (15g) |
|
| 97 (17c) | ||||||
|
| ||||||
| 3 | C4H9 | C10H21 | 49 | 13c: 14c = 7.7:1 | 52 (17d) |
|
|
| ||||||
| 4 | C2H5 | C10H21 | 58 | 13d: 14d = 6:1 | 25 (15f) |
|
| 99 (17a) | ||||||
|
| ||||||
| 5 | Me E/Z | C10H21 | 20 | 13e:14e = 1.5:1 | 38 (15e) |
|
| 24 (16b) | ||||||
|
| ||||||
| 6 | C8H17 | C8H17 | 42 | 13f:14f = 8 4:1 | ref. 22 |
|
|
| ||||||
| 7 | C6H13 | ally1 | 60 | 13g:14g = ND | 68 (15j) |
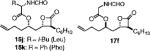
|
| 46 (15k) | ||||||
| 55 (17f) | ||||||
|
| ||||||
| 8 | C6H13 | trans-croty l | 62 | 13h:14b = 9:1 | 45 (15h) |
|
| ND (15i) | ||||||
| 57 (17g) | ||||||
| ND (17h) | ||||||
|
| ||||||
| 9 | C6H13 | H | 42 | 13i:14i = 7.1:1 | 52 (17d) |
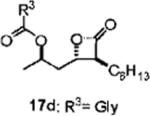
|
|
| ||||||
| 10 | C6H13 | TsO | 42 | 13j:14j = 7.7:1 | 58 (17e) |
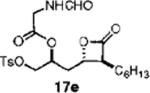
|
|
| ||||||
| 11 | C6H13 | C10H21 | NDa | 13j:14j= 3:1 | 51 (17i) |
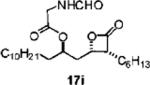
|
ND = not determined.
Includes yield for TBS deprotection step.
