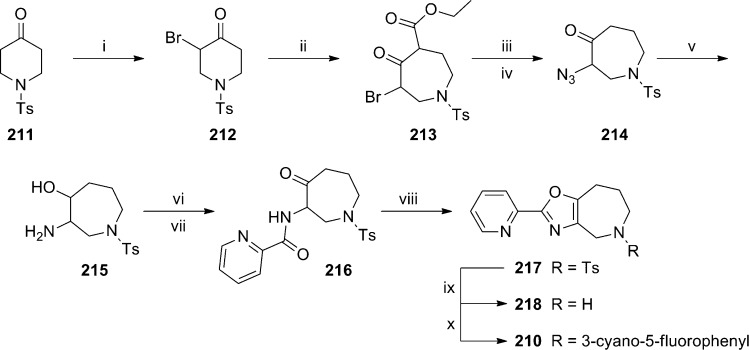
Scheme 17.
(i) Br2, CH2Cl2, −5 to −2 °C, 98%; (ii) ethyl diazoacetate, BF3·OEt2, CH2Cl2, −5 to 0 °C, 72%; (iii) 3 N aq. HCl, dioxane, 100 °C, 90%; (iv) NaN3, DMF, 80%; (v) LiAlH4, THF, 0 °C, 52%; (vi) picolinic acid, EDC, HOBt, NEt3, CH2Cl2, 80%; (vii) Dess–Martin periodinane, CH2Cl2, 92%; (viii) Burgess’ reagent, THF, 150 °C, μw, 45 min, 78%; (ix) 48% aq. HBr, 100 °C, 74%; (x) 3-bromo-5-fluorobenzonitrile, Pd2(dba)3, XANTPHOS, KOtBu, PhMe, μw, 100 °C, 22%.