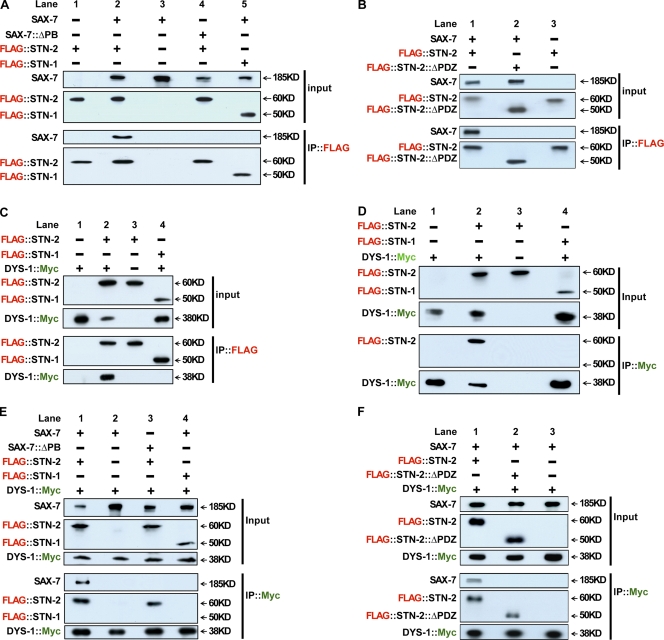
Figure 7.
STN-2 acts as a linker protein that bridges SAX-7 to DYS-1, as determined by co-IP assays in HEK293 cells. (A and B) Neurofascin::SAX-7CT (labeled SAX-7) interacts with STN-2, as shown in A (lane 2) and B (lane 1). This interaction depends on the SAX-7 PB sequence (A, lane 4) and the STN-2 PDZ domain (B, lane 2). This interaction between SAX-7 and STN-2 is specific because SAX-7 does not interact with STN-1 (A, lane 5). (C and D) The C-terminal end of DYS-1 (amino acid residues 3,402–3,674) interacts with STN-2, but not STN-1, as DYS-1 coimmunoprecipitates with STN-2 (C, lane 2) but not with STN-1 (C, lane 4). The reverse IP shows that STN-2 (D, lane 2), but not STN-1 (D, lane 4), coimmunoprecipitates with DYS-1. (E and F) Although SAX-7 does not coimmunoprecipitate with DYS-1 (E, lane 2; and F, lane 3), SAX-7 does so when STN-2 is present (E, lane 1; and F, lane 1), suggesting this tripartite complex requires STN-2 as a linking molecule. Co-IP of SAX-7 with STN-2 and DYS-1 requires the PB sequence of SAX-7 (E, lane 3) and the PDZ domain of STN-2 (F, lane 2).