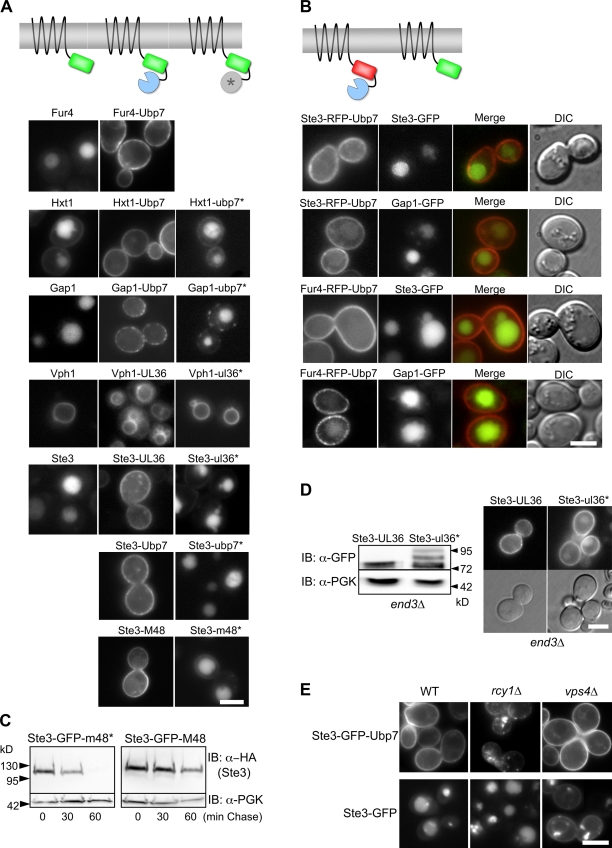
Figure 3.
Fusing cargo to the catalytic domain from deubiquitinating enzymes blocks ubiquitination and trafficking to the vacuole. (A) Schematic of DUb catalytic domain fusion proteins (top). Localization of the indicated GFP-tagged proteins fused to either enzymatically active or inactive (*) catalytic domains of Ubp7, HSV-1 UL36, and mCMV M48 (bottom). (B) The effect of DUb fusion proteins (Ste3-RFP-Ubp7 or Fur4-RFP-Ubp7) on coexpressed Gap1-GFP or Ste3-GFP. (C) Cycloheximide chase with cells expressing HA-tagged Ste3-GFP-m48* and Ste3-GFP-M48, which carried active and inactive DUb domains, respectively. Aliquots were removed at the indicated times after cycloheximide addition (100 µg/ml) and immunoblotted for HA and PGK. (D) Ste3-GFP-UL36 or Ste3-GFP-ul36* (with an inactive catalytic domain) were expressed in end3Δ cells and immunoblotted with anti-GFP antibodies (left) or localized (right). (E) Localization of Ste3-GFP or Ste3-GFP-Ubp7 in wild type, rcy1Δ cells, and vps4Δ cells . Bars, 5 µm.