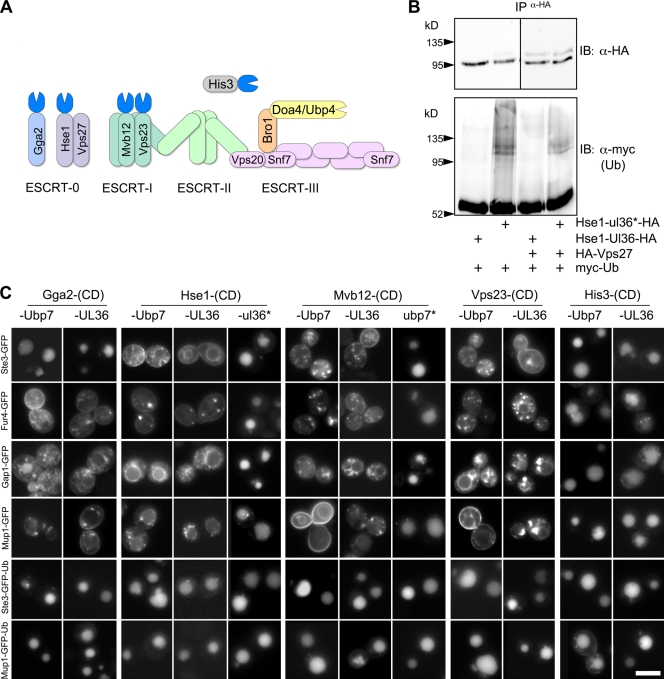
Figure 4.
Fusion of DUbs to ESCRT proteins inhibits their ubiquitination but allows vacuolar sorting of Ub fusion cargo proteins. (A) Schematic of the ESCRT sorting apparatus showing subunits fused to different DUb catalytic domains. (B) Cells expressing myc-Ub and either Hse1-UL36-HA or Hse1-ul36*-HA (inactive) were transformed with vector alone or with plasmids expressing HA-Vps27. Lysates were immunoprecipitated (IP) with anti-HA antibodies. Immunoprecipitates were immunoblotted (IB) for HA (top) to confirm isolation of HA-tagged proteins; immunoprecipitates were immunoblotted with anti-myc to assess ubiquitination. Fusion of active UL36 abolished ubiquitination of either HA-tagged Hse1 or Vps27 proteins. Black lines indicate that dividing lanes have been spliced out. (C) ESCRT subunits, GGA, or His3 fused to the indicated active (Ubp7 or UL36) or inactive (ubp7* or ul36*) catalytic domains (CD) were expressed in wild-type cells. Cells coexpressed the indicated GFP-tagged MVB cargo. Bar, 6 µm.