Figure 3.
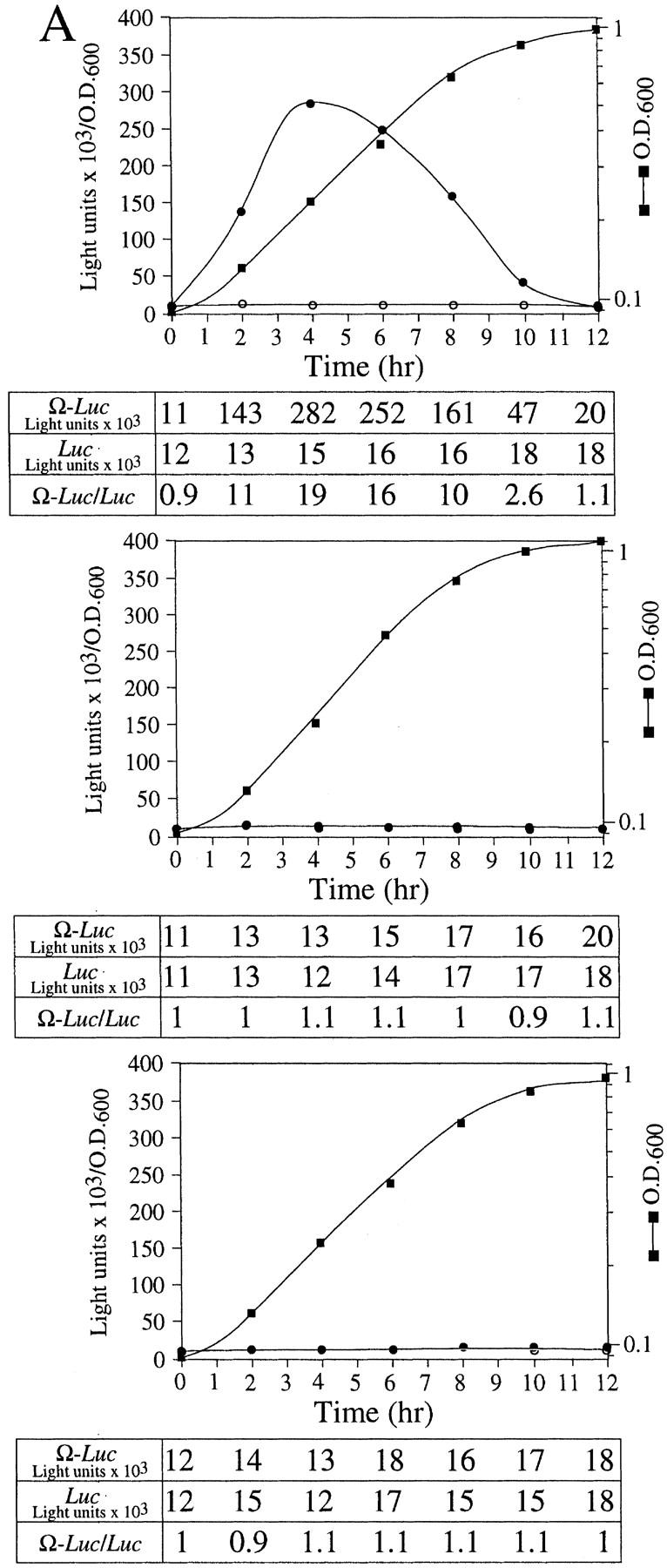
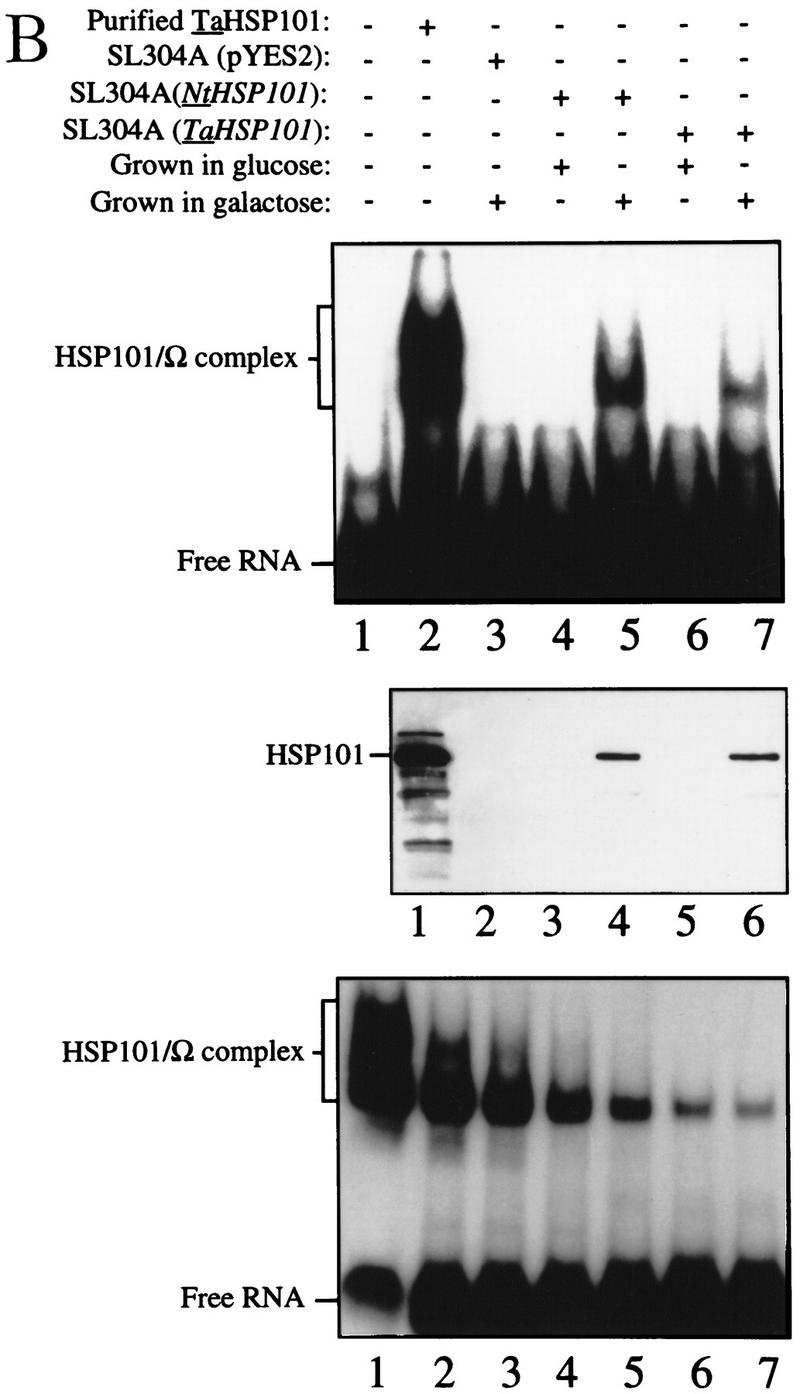
NtHSP101 specifically enhancesexpression from an mRNA when Ω is present as the 5′ leader. (A) Expression from pTPI–Ω-luc (•) or pTPI–luc (○) was followed in SL304A transformed with pGAL1–NtHSP101 (top and middle) or pYES2 (bottom) to examine the regulatory role of NtHSP101 during translation. Transformants were first grown to late-exponential stage (whereupon expression from pTPI–Ω-luc and pTPI–luc was equivalent) and then inoculated into synthetic galactose medium (top and bottom) or synthetic dextrose medium (middle) at the zero time point. Luciferase expression was measured at time points during the growth cycle, normalized to the OD (right) during growth. The expression from pGAL1–Ω-luc, pGAL1–luc, and their expression ratio (i.e., NtHSP101 activity as indicated by the ratio, Ω-luc/luc) are shown below each panel. (B) Tobacco and wheat HSP101 retain RNA-binding activity when expressed in yeast. (Top) SL304A containing pGAL1–NtHSP101 or pGAL1–TaHSP101 were grown in galactose or glucose and crude extracts used in RNA gel shift binding assays with radiolabeled Ω RNA. SL304A(pYES2) was used as a negative control and purified HSP101 was included as a positive control. (Middle) Western analysis of crude extracts from SL304A (containing either pGAL1–NtHSP101, pGAL1–TaHSP101, or pYES2) by use of anti-p102 (i.e., anti-HSP101) antibodies. The lanes correspond to the extracts as indicated at top. The lower molecular weight bands in lane 1 are degradation products of HSP101 that are observed when a high level of the purified protein is analyzed. (Bottom) Titration of HSP101 protein in the Ω RNA gel shift binding assay. The concentration of purified HSP101 used in each binding reaction was decreased twofold in each succeeding lane, whereas the concentration of Ω RNA was held constant.
