Figure 5.
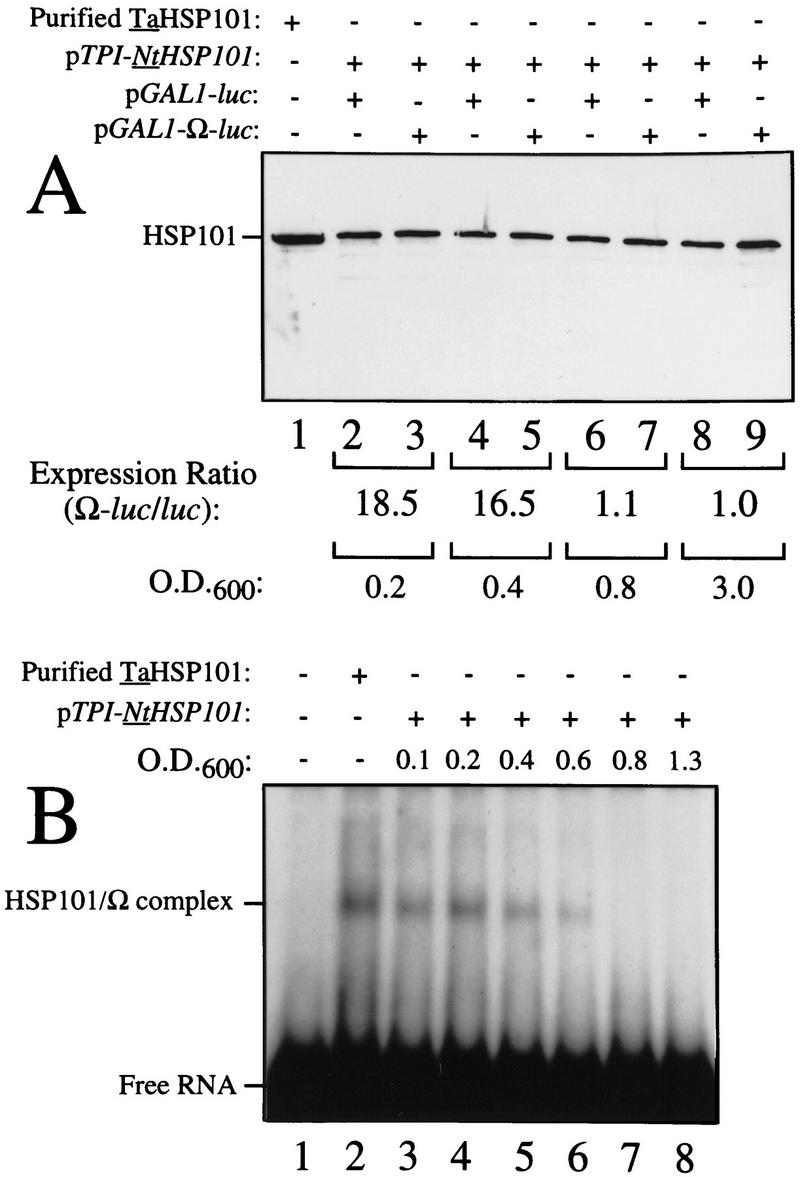
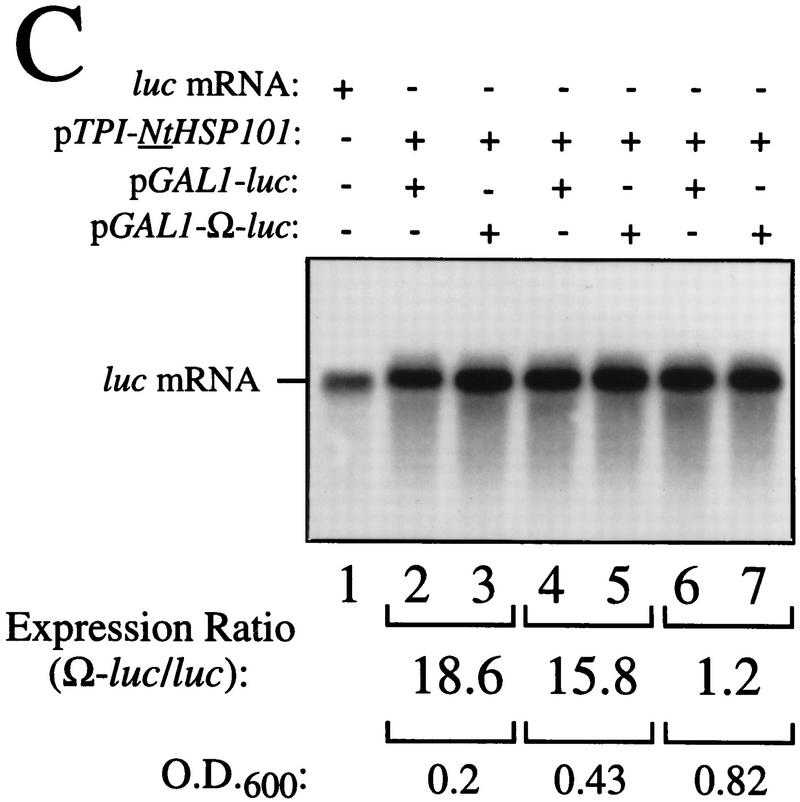
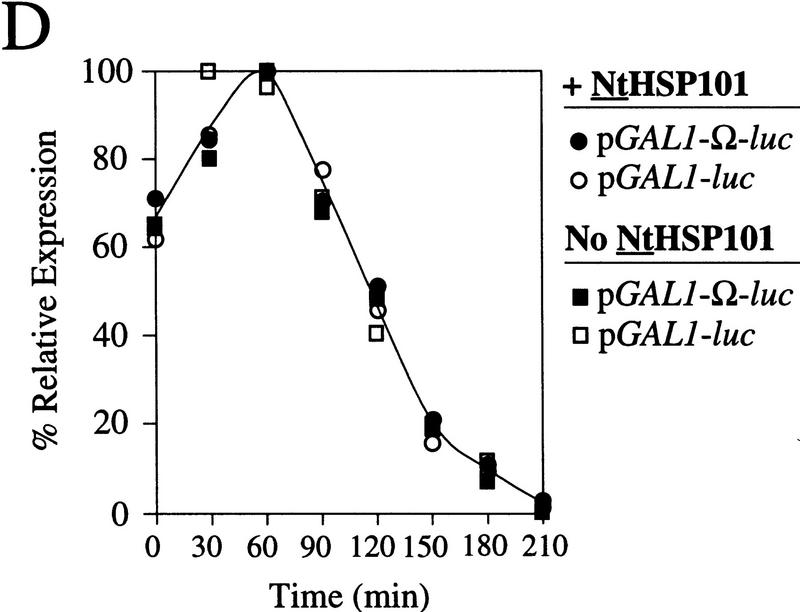
HSP101 RNA-binding activity is regulated during yeast growth without corresponding changes in luciferase mRNA or protein stability. (A) Western analysis of extracts from early, mid, or late-exponential or stationary cells of SL304A(pTPI–NtHSP101) containing either pGAL1–luc or pGAL1–Ω-luc grown in SGM. The expression ratio of Ω-luc/luc at each OD indicated is shown below each pair of lanes. Purified HSP101 from wheat germ (TaHSP101) was loaded in lane 1 as a positive control. (B) NtHSP101 RNA binding to radiolabeled Ω was assayed by RNA gel shift analysis by use of crude extracts of early, mid-, or late exponential SL304A(pTPI–NtHSP101) cells grown in SGM to the OD indicated. Purified TaHSP101, whose RNA-binding properties were characterized previously (Tanguay and Gallie 1996), was included in lane 2 as a positive control. (C) Northern analysis of luc mRNA levels from either pGAL1–Ω-luc or pGAL1–luc in early, mid-, or late-exponential SL304A(pTPI–NtHSP101) cells grown in SGM. Total RNA was used for the analysis and in vitro-synthesized luc mRNA (lane 1) was included to indicate the size of the mRNA. The OD of the cells and expression ratio (i.e., translation from Ω-luc relative to luc mRNA) is indicated below each pair of lanes representing each growth stage examined. (D) The effect of NtHSP101 on luciferase protein stability was determined by switching early exponential SL304A(pTPI–NtHSP101, ○,•) or SL304A(pYX232, □,█) containing either pGAL1–Ω-luc or pGAL1–luc from SGM to SDM and the level of luciferase measured at time points following the transcriptional repression of the GAL1 promoter. Equal volumes of cell culture were used for the measurements to account for the dilution of luciferase protein during cell growth and division. The amount of luciferase activity is shown relative to the level (defined as 100%) present in the cells immediately following introduction into SDM.