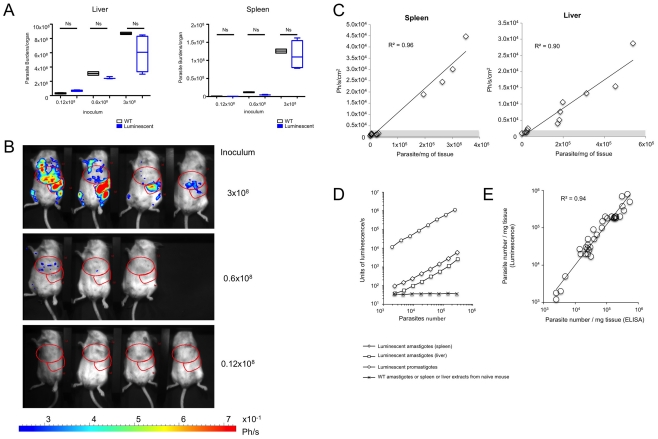
Figure 1. Infectivity of LUC-parasites and sensitivity of bioluminescence in vivo and ex vivo.
A. Infectivity of Luc-parasites as compared to L. infantum WT parasites. Both luciferase transgenic and wild type L. infantum stationary phase promastigotes establish themselves in BALB/c mice. Groups of 4 BALB/c mice were given either 0.12×108 to 3×108 stationary phase luciferase-transgenic or wild type L. infantum. One month later, mice were imaged, the bioluminescence being recorded before their sacrifice. L. infantum burdens were estimated by ELISA in the liver and the spleen. Burdens were calculated as parasite/mg organ x organ weight (in mg). Data representative of three experiments are presented as box whisker plots. For statistical analysis Man Whitney Wilcoxon test was performed and did not show any significant difference between parasite loads of mice inoculated with WT or luciferase parasites; ns = not-significant. B. Bioimaging of BALB/c mice one month post the IV inoculation of luciferase transgenic L. infantum. Mice inoculated with LUC-parasite and with parasite loads depicted in figure 1A, were given luciferin via IP. The photon emission was recorded once the anesthetized mice were deposited in the imaging chamber of the Photon Imager. Red zones are ROIs that delineate the liver or spleen. C. Sensitivity of BLI. Luminescence (photon/s/cm2) was recorded in ROIs corresponding to liver and spleen of the 11 mice infected with LUC-parasites depicted in figure 1A. Luminescence recorded in ROIs was plotted versus parasite density measured by ELISA as described in figure 1A to generate regression curves. The threshold sensitively of BLI calculated as three times luminescence of naïve mice (indicated by the gray zone) was 20,000 to 40,000 parasites/mg for spleen and liver respectively. D. Ex-vivo quantification of parasite loads by bioluminescence. Luminescence of equal numbers of the LUC-parasite under exponentially growing promastigote form or liver and spleen amastigotes was analyzed using a luminometer. Equal numbers of liver and spleen amastigotes from mice infected with WT promastigotes were used as specificity control. Sensitivity of ex vivo analysis of parasite density by bioluminescence (1,000 to 6,000 amastigotes/mg for spleen and liver respectively) was calculated as parasite numbers corresponding to twice luminescence background values. E. Accuracy of ex vivo bioluminescence analysis for estimation of parasite density. Liver and spleen samples from 18 IV infected BALB/c mice were detergent extracted and assayed both by ELISA and bioluminescence. Values generated by both techniques were plotted to generate regression curves.