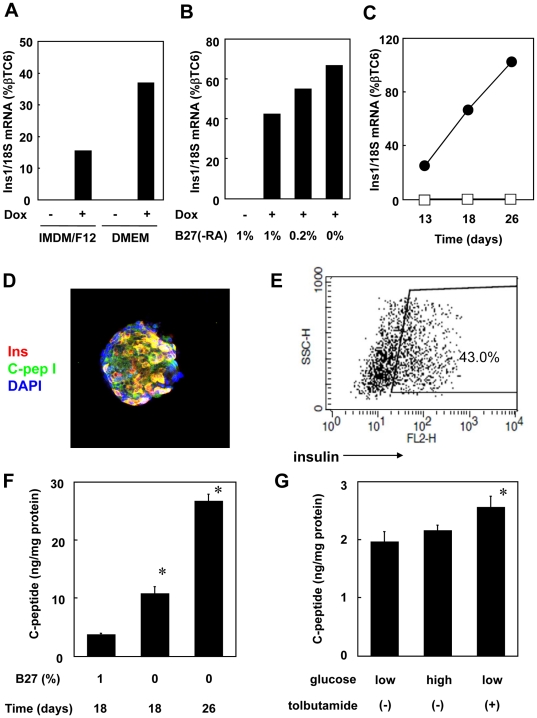
Figure 6. DMEM media improves pancreatic differentiation of mouse ES cells.
Tet-pdx1/ngn3 ES cells were cultured under Protocol #3 for 6 days, with Dox-induction of Pdx1 and Ngn3 expression starting at day 4. A) From day 6 through day 18 EBs were cultured according to Protocol #3 (“IMDM/F12”) or in medium in which the IMDM/F12 was replaced with DMEM (“DMEM”) as indicated (Protocol #4), and Ins1 mRNA was quantitated at day 18. B) EBs were cultured according to Protocol #4 for the first 6 days and with Dox-induction of Pdx1 and Ngn3 expression starting at day 4. From day 6 through day 18 various concentrations of B27(−RA) were added as indicated. Ins1 mRNA was quantitated at day 18. C) EBs were cultured according to Protocol #4 for 6 days, with Dox-induction of Pdx1 and Ngn3 expression starting at day 4. From day 6 through day 26 the medium was only DMEM with BSA without B27(−RA). Ins1 mRNA was quantitated from day 13–26. (D–G) Tet-pdx1/ngn3 ES cells were cultured according to Protocol #4 for 24 days with Dox induction starting at day 4. D) EBs were replated at day 24 and cultured in DMEM (BSA). At day 26, Insulin was visualized with a Cy3-conjugated 2nd antibody (red) and C-peptide was visualized with a FITC-conjugated 2nd antibody (green). Nuclei were stained with DAPI (blue). Magnification was with a 400× objective. E) EBs, prepared as in panel D, were trypsinized to make single cell suspensions and cytoplasmic insulin was stained and analyzed by FACS. F) EBs were cultured under Protocol #4 for 6 days, with Dox-induction of Pdx1 and Ngn3 expression starting at day 4. From day 6 through day 18 only one group was supplemented with B27(−RA) (1%) as indicated. On either day 18 or day 26 the media was replaced with fresh DMEM with BSA. Supernatants were harvested after 24 hours, and C-peptide was measured by RIA. The data presented are mean of three independent experiments; the error bars represent the SEM. p<0.05 as compared with 1% B27 at day 18. G) EBs were prepared as described in panel F. On day 26 the media was replaced with HKRB buffer containing either low glucose media (2 mM) or high glucose media (20 mM) with or without tolbutamide. Supernatants were harvested after 1 hour and C-peptide was measured by RIA. The data presented are mean of three independent experiments; the error bars represent the SEM. p<0.05 as compared with low glucose without tolbutamide.