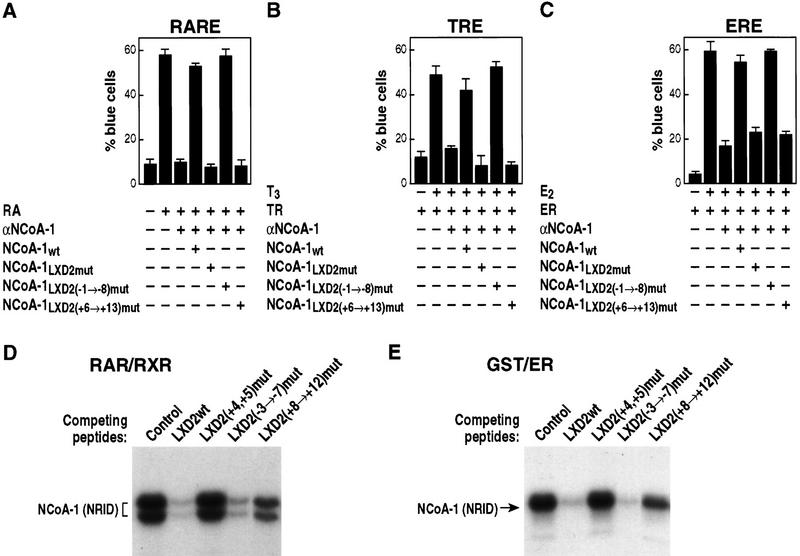
Figure 4.
Carboxy-terminal flanking regions dictate specificity of LXXLL domain function. Nuclear microinjection studies in Rat-1 cells were performed with NCoA-1/SRC-1 proteins in which LXD2-flanking residues (−1 → −8) or (+6 → 13) were mutated to alanine and were evaluated RAR, TR, and ER, as shown in A, B, and C, respectively. Results of the average ± s.e.m. of two sets of nuclear microinjected cells; three independent experiments gave similar results. (D) Avidin–biotin DNA complex assay with thrombin-cleaved, bacterially expressed RARβ and RXRα proteins were bound to biotinylated direct repeat core sequence spaced by 5 bp (DR+5) oligonucleotide, and the NRID of NCoA-1/SRC-1 (amino acids 700–763) was prepared as a 32P-labeled bacterial protein. Competition was assessed with wild-type LXD2 (21-mer) synthetic peptides (LXD2wt), or peptides containing alanine substitutions in the indicated amino acids used in excess (1 μm) to compete for binding. Binding of the NCoA-1 NRID indicates the efficiency of the peptide competition; with more binding indicating loss of function, as observed with mutation of L4 and L5. (E) Similar analysis performed with a bacterially expressed GST–ER carboxy-terminal protein, to evaluate the effects of residue substitution. Mutation of +8 through +12 caused considerable loss of function (i.e., less competition).