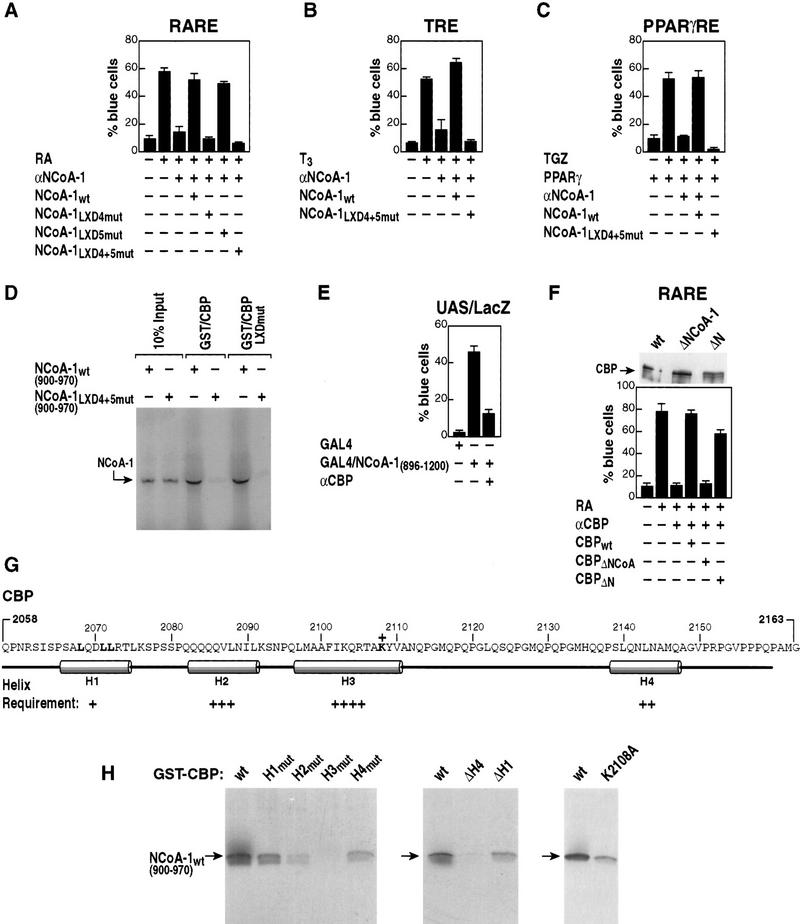
Figure 7.
Role of LXDs in the CBP/p300-interaction domain of NCoA-1/SRC-1 in interaction and receptor activation function. (A) The LXD motifs (4, 5; Fig. 1A) were mutated to place alanines in positions 2, 3, 4, 5 alone, or together, and evaluated for function on RAR-dependent gene activation. Mutations of LXD4 or LXD4 and LXD5 abolished the ability of SRC-1/NCoA-1 to function in retinoic acid-dependent activation events in Rat-1 single cell nuclear microinjection studies. (B,C) The requirement for the LXD4 and LXD5 in the CBP/p300-interaction domain of NCoA-1/SRC-1 is demonstrated for coactivation of the TR and PPARγ. (D) Role of LXDs in the interactions between 35S-labeled NCoA-1/SRC-1 CBP-interaction domain and CBP (wild type) or CBP in which the CBP LXD is mutated (LXXLL → LAAAA; CBP LXDmut). The mutation of LXD4 and LXD5 virtually abolished interactions by GST pull-downs, but mutation of the CBP LXXLL motif in the interaction domain did not affect interactions. (E) Gal4–NCoA-1 (896–1200) fusion protein activates transcription from the UAS p36 promoter (Torchia et al. 1997) and was blocked by addition of anti-CBP IgG (Kamei et al. 1996). (F) Effect of deletion of the NCoA-1 interaction domain of CBP on function of CBP in RAR activation. Anti-CBP IgG (Kamei et al. 1996) was used to block RAR activation, and CMV-expression vectors encoding wild-type CBP, CBPΔN (Δ 2–468), or CBP ΔNCoA-1 (Δ 2098–2163) were evaluated for their ability to rescue. The three CBP protein variants were expressed at comparable levels in transcripted cells as detected by anti-Flag IgG (top). (G) Predicted structure of the CBP region interacting with SRC-1/NCoA-1, with helix 3 as a predicted hydrophobic helix. (H) Requirements of each helix were tested by mutation to break the helix (helix 1, QDLL → PDPG; helix 2, QQQV → PQPG; helix 3, FIKQ → PIPG; helix 4, NLNA → PLPG), or by helix removal (ΔH4, amino acids 2058–2133; ΔH1, amino acids 2078–2163). Regions were tested in vitro for their ability to interact with amino acids 900–970 of NCoA-1/SRC-1, 35S-labeled by in vitro transcription and translation.