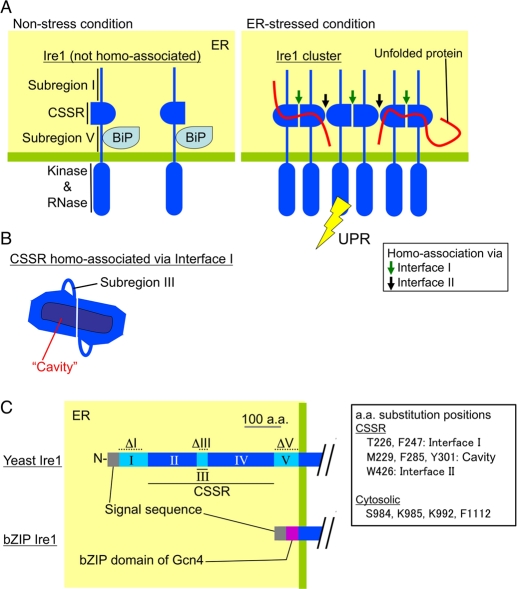
FIGURE 1:
Current model for structure and function of the luminal domain of S. cerevisiae Ire1. The luminal domain of yeast Ire1 can be divided into five subregions. Subregions I (aa 32–111), III (aa 243–272), and V (aa 455–524) are loosely folded, while subregions II (aa 112–242) and IV (aa 273–454) form the tightly folded CSSR (Kimata et al., 2004; Oikawa et al., 2005; Credle et al., 2005). (A) Under nonstress conditions, BiP associates with subregion V (Kimata et al., 2004). Dissociation of BiP from Ire1 leads to cluster formation of Ire1, the structural basis of which is two different modes of CSSR homoassociation (homoassociation via interfaces I and II; Credle et al., 2005; Kimata et al., 2007). Unfolded proteins then directly interact with the Ire1 cluster, causing full activation of Ire1 (Kimata et al., 2007). (B) The CSSR dimer associated via interface I forms a cavity-like structure, by which unfolded proteins may be captured (Credle et al., 2005). (C) The dashed lines indicate the positions of amino acid residues deleted in the ΔI (aa 32–91), ΔIII (aa 253–272), and ΔV (aa 463–524) mutations. The bZIP mutant of Ire1 carries the bZIP domain of Gcn4 instead of subregions I to V.