Figure 3.
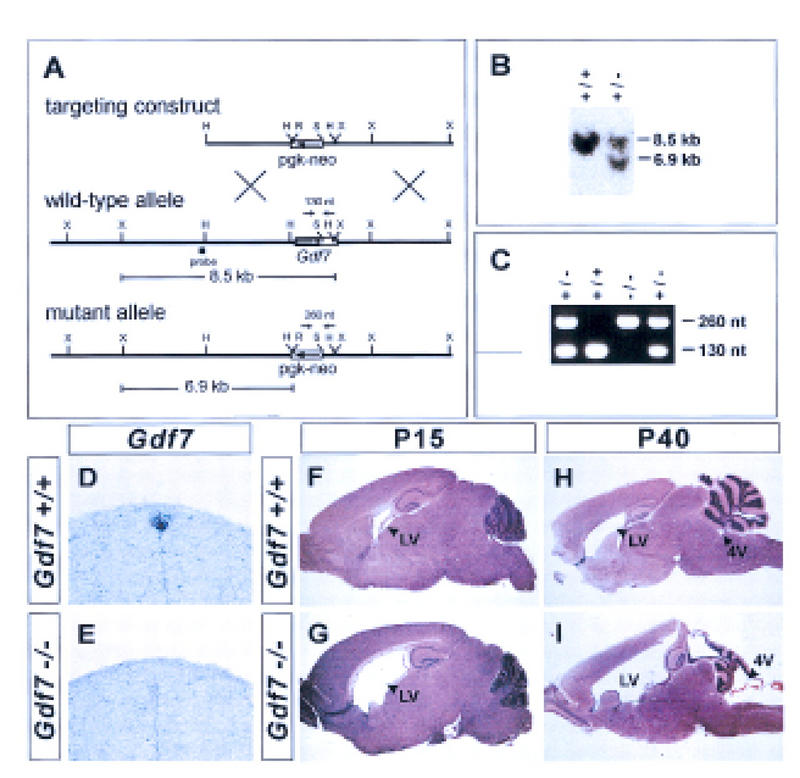
Generation of a Gdf7-null allele and hydrocephalic abnormalities in Gdf7 mutant mice. (A) Strategy for targeted disruption of the mouse Gdf7 locus by homologous recombination. The diagram indicates the position of the Gdf7 exon encoding half of the pro-domain and the entire mature carboxy-terminal region (coding region, solid rectangle; 3′ UTR, open rectangle). Restriction sites: (H) HindIII; (R) EcoRV; (S) SphI; (X) XbaI. (B) Southern blot analysis of DNA from ES clones digested with XbaI and EcoRV and probed with the fragment indicated in A. The presence of the wild-type allele is indicated by an 8.5-kb band and the disrupted allele by a 6.9-kb band. (C) PCR analysis of DNA from progeny of heterozygote matings with primers indicated in A. The wild-type allele is indicated by a 130-bp band and the mutant allele by a 260-bp band. (D,E) In situ hybridization of wild-type (D) and Gdf7m1 homozygous mutant (E) embryos with a Gdf7 coding region probe. Hybridization was not detected in the roof plate in Gdf7m1 homozygous mutant embryos. (F–I) Parasagittal sections of brains from wild-type (F,H) and Gdf7m1 homozygous mutant (G,I) mice stained with hematoxylin/eosin. Twenty-five percent of Gdf7 mutant brains (n = 8) analyzed at postnatal (P) day 15 showed a marked dilation of the lateral ventricles (LV). No obvious defects in morphology of the cerebellum and cerebral cortex were detected at this age. By P40, a severe hydrocephalus that is associated with dilation of the fourth (4V) and lateral ventricles (cf. H and I) was observed in 25%–37% of Gdf7 mutants (see Table 1). Hydrocephalic mutant animals showed considerable variation in the extent to which the different ventricles were enlarged. In some mutants (I) the fourth ventricle was dilated, and the organization and foliation of the cerebellum was disrupted. In all affected brains examined, however, the cerebral cortex was thinned and the hippocampus was displaced dorsally. Despite these abnormalities, histological analysis showed that the cellular architecture of the cerebellum and cerebral cortex was grossly normal in Gdf7-null mutants (data not shown). The late onset of brain defects and the relatively normal cellular organization in Gdf7 mutants suggest that the disruption of brain morphology is a secondary consequence of hydrocephalus. The expression of Gdf7 in the fourth ventricle choroid plexus (Fig. 1C) raises the possibility that defects in choroid plexus development may underlie the hydrocephalus observed in Gdf7m1 mutant mice. Histological analysis indicated that the fourth ventricle choroid plexus epithelium was still present in affected Gdf7m1 mutant mice and expression of Msx1, Bmp6, and Bmp7 in the choroid plexus appeared normal (data not shown). Thus, the cellular basis of the hydrocephalus is unclear, but it is likely to reflect a requirement for Gdf7 function in choroid plexus cells.
