Figure 3.
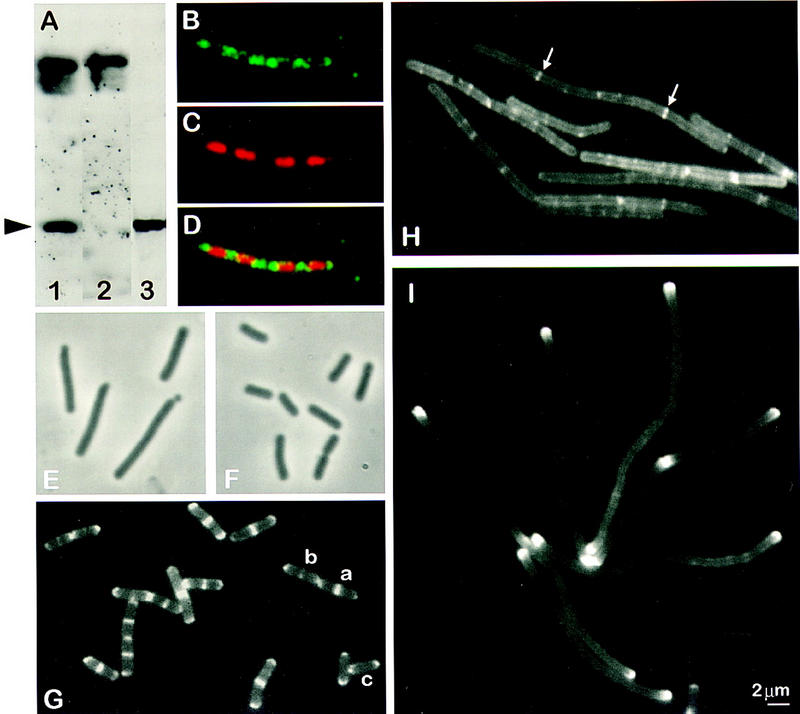
Subcellular localization of MinD and its dependence on divIVA and ftsZ. (A) Western blot, performed after SDS-PAGE (10%) and electrotransfer, demonstrating affinity purification of the anti-MinD antibodies. Crude anti-MinD antiserum was used to probe vegetative extracts of wild-type (SG38; lane 1) and minD (1901; lane 2) cells. After affinity purification, the anti-MinD antiserum recognized a single band in extracts of wild-type cells (SG38; lane 3). The arrowhead indicates the position of MinD protein. (B–D) Immunofluorescence micrographs showing detection of MinD at the cell poles and between nucleoids. The cells were stained for MinD protein (B) and DNA with DAPI (C), with the two images merged in D. (E,F) Phase-contrast images showing complementation of ΔminD by gfp–minD. Strain 1981 (ΔminD Pxyl–gfp–minD) grown in S medium (E) or S medium containing 0.5% xylose (F). (G) Fluorescence images showing the distribution of GFP–MinD in growing cells (S medium) of B. subtilis strain 1981. Examples of cells with different patterns of GFP–MinD are labeled a–c (see text). (H) Dependence of targeting of GFP–MinD to the poles on divIVA. Strain 1984 (ΔminD divIVA− Pxyl–gfp–minD) was grown in S medium and gfp–minD expression was induced by the addition of 0.5% xylose. (I) Polar targeting of GFP–MinD in FtsZ− filaments.Strain 1979 (ΔminD Pspac–ftsZ Pxyl–gfp–minD) was grown in S medium containing 0.5 mm IPTG and 0.5% xylose, and ftsZ was depleted by removing the IPTG.
