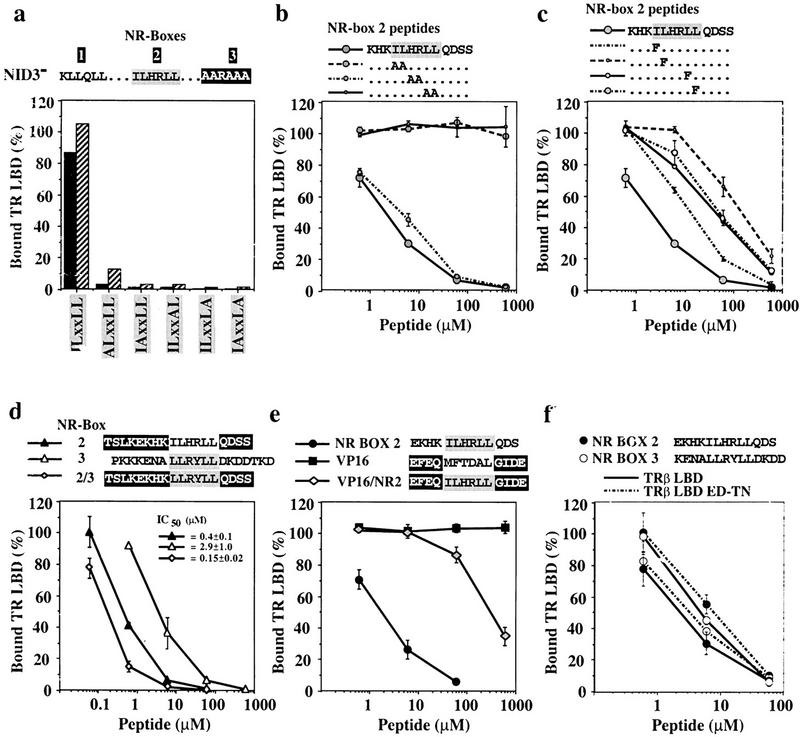
Figure 5.
(a) Individual leucine residues of the LxxLL motif are crucial for binding of GRIP1 NID to TRβ LBD. Shown are 1.6 μm (solid bars) or 4.0 μm (hatched bars) of glutathione–agarose-bound GST–NID3− or variants containing alanine substitutions of individual hydrophobic residues of the NR-box 2 ILxxLL motif (ALxxLL: I689A; IAxxLL: L690A; ILxxAL: L693A; ILxxLA: L694A; IAxxLA: L690A + L694A) were incubated with labeled TRβ LBD in the presence of 10 μm T3 (mutations are in boldface type). The amount of bound receptor is relative to the receptor input. These are the results of a representative experiment. (b,c) Pairwise or single conservative substitutions of the ILxxLL leucine residues drastically reduce the affinity of NR-box 2 peptides for TRβ LBD. Interaction of 1.6 μm glutathione–agarose-bound NID3− with 10 nm labeled TRβ LBD + 10 μm T3 was competed with increasing concentrations of variants of the NR-box 2 peptide KHKILHRLLQDSS, containing either pairwise alanine substitutions KHKAAHRLLQDSS, KHKILAALLQDSS, KHKILHRAAQDSS (b), or single phenylalanine substitutions of conserved residues of the hLxxLL motif (KHKFLHRLLQDSS, KHKIFHRLLQDSS, KHKILHRFLQDSS, KHKILHRLFQDSS) (c) (mutations are in boldface type). The amount of bound receptor is relative to the amount of retained receptor in the absence of peptide. The data represent the average and standard deviation of three independent experiments. (d,e) Sequences adjacent to the LxxLL motif affect the affinity of the TRβ LBD:NR-box 2 interaction. Labeled TRβ LBD (10 nm) was incubated with 1.6 μm glutathione–agarose-bound GST–NID3− in the presence of 10 μm T3 and increasing concentrations of peptides containing NR-box 2 (solid triangle), NR-box 3 (open triangle), and the LLRYLL motif of NR-box 3 in the context of flanking sequences from NR-box 2 (shaded diamond) (d), or NR-box 2 (solid circle), VP16 (solid square), and the ILHRLL motif of NR-box 2 in the context of the adjacent sequences from the VP16 peptide (shaded diamond) (e). The amount of bound receptor is relative to the amount of retained receptor in the absence of peptide. The data and IC50 values represent the average and standard deviation of three independent experiments. (f) Labeled TRβ LBD (solid line) or TRβ LBD ED–TN (broken line) (10 nm) was incubated with 1.6 μm glutathione–agarose-bound GST–NID3− in the presence of 10 μm T3 and increasing concentrations of peptides containing NR-box 2 (solid circle), or NR-box 3 (open circle). The amount of bound receptor is relative to the amount of retained receptor in the absence of peptide. The data represent the average and standard deviation of three independent experiments.