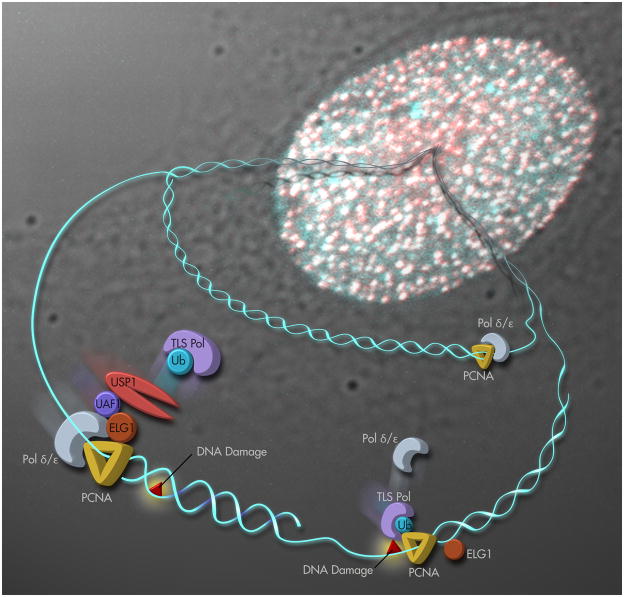
Figure 2.
A model for the role of ELG1 in PCNA deubiquitylation. Upon encountering DNA damage, the replication machinery becomes stalled. PCNA is then monoubiquitylated, and a TLS polymerase is recruited to the damage site to replace the replicative polymerase (Polδ/ε) and bypass the lesion. Once bypass has occurred, ELG1, which becomes concentrated at stalled forks with distinct foci structures in the nucleus, binds to PCNA and recruits the USP1/UAF1 complex. USP1 deubiquitylates PCNA, displacing the TLS polymerase and allowing Polδ/ε to resume normal replication.