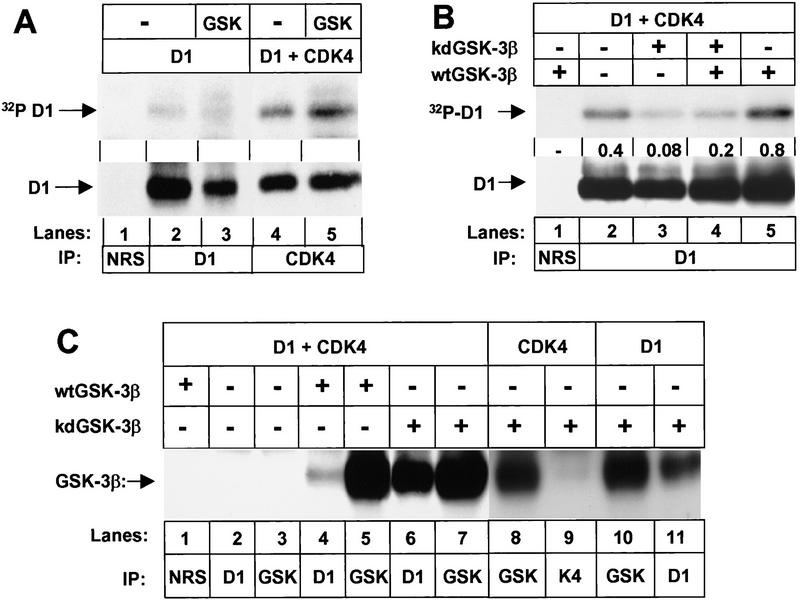
Figure 2.
GSK-3β binds to D1 subunits and preferentially phosphorylates cyclin D1 in complexes with CDK4. (A) Sf9 cells infected with baculoviruses encoding cyclin D1 or cyclin D1 plus CDK4 with or without wild-type GSK-3β as indicated were metabolically labeled with [32P]orthophosphate. Lysates were subjected to precipitation with NRS (lane 1), antibody to cyclin D1 (lanes 2,3), or antiserum specific for the carboxyl terminus of CDK4 (lanes 4,5) as indicated. Phosphorylated proteins were resolved on denaturing polyacrylamide gels and transferred to a nitrocellulose membrane. Following autoradiography, the membrane was blotted with the antibody to cyclin D1 (bottom) and sites of antibody binding were visualized by enhanced chemiluminescence. (B) Sf9 cells infected with baculoviruses encoding cyclin D1 and CDK4 together with wild-type (wt) or kinase-defective (kd) GSK-3β were labeled with [32P]orthophosphate. Lysates were subjected to precipitation with NRS or anti-D1 as indicated, and phosphorylated proteins were resolved on a denaturing polyacrylamide gel followed by transfer to Immobilon-P membrane (autoradiographic exposure time 12 hr; top). Following autoradiography, the membrane was blotted with the antibody to cyclin D1 as in A. The relative ratio of 32P-labeled cyclin D1 versus total cyclin D1 in each lysate (densitometric scanning) is indicated between the two autoradiographs. (C) Sf9 lysates infected with baculoviruses encoding the proteins indicated were precipitated with the indicated antibodies and blotted with a monoclonal antibody to GSK-3β. Sites of antibody binding were visualized by enhanced chemiluminesence.