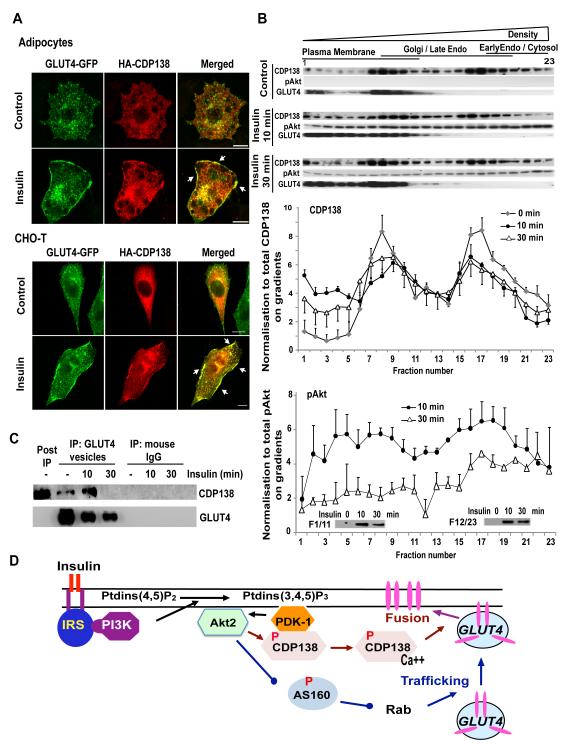
Figure 7. CDP138 is colocalized with GLUT4 at the PM (A) and dynamically associated with the PM fractions (B) and GLUT4 vesicles (C).
HA-CDP138-WT plasmid DNA was transfected alone into a CHO-T cell line stably expressing myc-GLUT4-GFP, or into adipocytes together with the myc-GLUT4-GFP vector. Forty-eight hr later, serum starved cells were treated with or without insulin for 10 min before immunofluorescent staining with anti-HA Ab, as described for Figure 5. Scare Bars: 10 μm. (B): Post nuclear supernatants from 3T3-L1 adipocytes treated with or without insulin 100nM were fractionated with iodixanol gradients (10-20-30% Optiprep) as described in S.I. Equal volumes of each fraction were resolved in 4-20% SDS-PAGE followed by immunoblotting with antibodies against CDP138, p-Akt and GLUT4. For quantification, the results were normalized to the total level of the indicated protein. Data are mean ± SEM of 3 independent experiments. (C): GLUT4 vesicles were enriched by immunoabsorption with anti-GLUT4 Ab (1F8) from adipocytes after removal of nuclear fraction as described in S.I. Samples were then immunoblotted with anti-CDP138 and anti-GLUT4 antibodies. Images are representative of three independent experiments (A, B & C). (D): Akt substrate CDP138 is a potential link between Akt2 activation and GSV - PM fusion. See also Figure S6.