Abstract
Genetic modulation of glucocorticoid receptor (GR) function in the brain using transgenic and gene knockout mice has yielded important insights into many aspects of GR effects on behavior and neuroendocrine responses, but significant limitations regarding interpretation of region-specific and temporal requirements remain. Here, we summarize the behavioral phenotype associated with two knockout mouse models to define the role of GRs specifically within the forebrain and amygdala. We report that forebrain-specific GR knockout mice exhibit impaired negative feedback regulation of the hypothalamic-pituitary-adrenal (HPA) axis and increased despair- and anxiety-like behaviors. In addition, mice with a disruption of GR specifically within the central nucleus of the amygdala (CeA) are deficient in conditioned fear behavior. Overall, these models serve as beneficial tools to better understand the biology of GR signaling in the normal stress response and in mood disorders.
Keywords: glucocorticoid receptor genetic disruption, forebrain, amygdala, anxiety, depression
1. Introduction
Stress, in the biological and psychological context, refers to the deviation of an organism from its original set-point in response to an imbalance in homeostasis. In response to stress, glucocorticoids are released from the adrenal cortex and, in turn, activate the hypothalamic-pituitary-adrenal (HPA) axis, a major part of the neuroendocrine system that works to restore homeostasis. Glucocorticoid secretion is controlled through negative and positive feedback systems maintained at different structures throughout the CNS. Two types of intracellular receptors mediate the effects of circulating glucocorticoids (i.e. corticosterone in rodents, cortisol in humans): i) the type I mineralocorticoid receptor; reviewed elsewhere (Kolber et al., 2008b) and ii) and the type II GR (Beato et al., 1995, De Kloet et al., 1998). These receptors are ligand-binding transcription factors that belong to the nuclear hormone receptor superfamily and display distinct but overlapping expression patterns throughout the CNS (De Kloet et al., 1998). GR acts to modulate a wide range of neural functions, including stress responsiveness and cognition, by binding to glucocorticoid response elements (GREs) in DNA regulatory regions (De Kloet et al., 1998, Holsboer, 2000, Reul et al., 2000). This mode of operation leads to transactivation, or occasionally repression, of transcription. Alternatively, GR can interact directly with other transcription factors (e.g. NFκB and AP-1), independent of DNA binding, to alter transcription.
There are several lines of evidence suggesting that dysregulation of HPA axis activity, postulated to be caused by impaired GR signaling, may be a primary factor in the pathogenesis of affective disorders (Pariante, 2003, Steckler et al., 1999). These studies provide strong evidence in support of the hypothesis that altered GR activity is an etiologic factor underlying anxiety and depression. According to this hypothesis, mice with genetic alterations in GR neural circuitry would be expected to display alterations in emotional behavior and depression-like states (Urani & Gass, 2003).
Multiple strains of mice with targeted GR deficiencies have been created over the past 15 years. Mice with a global deletion of GR die shortly after birth due to impaired lung function preventing analysis of endocrine function and behavioral effects (Cole et al., 1995). Transgenic mice with reduced GR function have also been produced using antisense GR mRNA (Pepin et al., 1992). These mice show basal plasma adrenocorticotropic hormone (ACTH) and corticosterone levels similar to control animals but display major disturbances in HPA axis regulation which seem to be caused by defects in the pituitary CRH receptor system, sympathetic output and adrenal development (Barden et al., 1997). These mice also display decreased anxiety-like and despair-related behavior (Montkowski et al., 1995). While these findings implicate a role for GR in regulation of the HPA axis and behavior, this mutation affects global GR expression making it difficult to distinguish acute effects of GR deletion from developmental adaptations to chronic GR loss. Finally, Tronche et al. generated mice with a nervous system specific knockout of GR using the Cre/loxp recombination system (GRnes/cre) (Tronche et al., 1999). These mice display hyperactivity of the HPA axis, decreased anxiety and decreased levels of despair-like behaviors. However, due to the global deletion of GR throughout the CNS in these mice, specific analysis of neural circuitries have been hindered by GR deletion in areas of the brain that provide major feedback inhibition to the HPA axis. Moreover, the deletion from early in development together with long-standing glucocorticoid excess complicates analysis due to both the potential for establishment of compensatory pathways and motoric compromise resulting from steroid-induced muscle loss.
Here, we describe two potentially informative models of GR function specific to the amygdala and within the forebrain and summarize the behavioral phenotype of each (Table 1).
Table 1. Behavioral Phenotype of FBGRKO and CeAGRKO mice.
| Behavioral phenotvpe | FBGRKO | CeAGRKO |
|---|---|---|
| Anxiety |
× (OF) (LDP) (EPM) |
nc |
| Depression | + (reversible with imipramine) | n/a |
| Anhedonia | + | n/a |
| Fear conditioning | nc | deficit in contextual freezing attenuation of auditory-cued freezing |
| Hyperactivity |
X (OF) + (LDP [following 30 min of restraint]) + (EPM) |
nc |
n/a: not performed. (+): increase in behavioral domain as compared to control mice. (−): decrease in behavior as compared to control mice. (nc): no significant difference in relative behavior as compared to control mice. OF: open field testing, LDP: light/dark preference testing, EPM: elevated plus maze.
2. Generation of FBGRKO and CeAGRKO mice
FBGRKO
Mice with a primary disruption in GR specifically in the forebrain were generated to assess the role of forebrain GR on HPA axis regulation and emotional behavior (Boyle et al., 2005). Briefly, mice homozygous for a GRloxPneo allele (Brewer et al., 2003) were crossed with mice expressing a transgene in which Cre recombinase is expressed under the control of the forebrain-specific calcium/calmodulin-dependent kinase II (CaMKIIα) promoter (T50Cre) (Boyle et al., 2005, Tsien et al., 1996). Mice homozygous for the GRloxPneo allele expressing Cre recombinase result in forebrain-specific disruption of GR (FBGRKO). 90–100% of GR immunoreactivity is not lost in the forebrain until four-six months of age. In FBGRKO mice, GR expression is disrupted in the hippocampus, cerebral cortex, striatum, nucleus accumbens, caudate-putamen, and basolateral nucleus of the amygdala (BLA) but not in the central nucleus of the amygdala (CeA), paraventricular nucleus of the hypothalamus (PVN), thalamus or pituitary (Fig. 1a&b) (Boyle et al., 2006). Four to six-month-old male littermate knockout and control (Cre negative) mice were used for all experiments. Mice are of a mixed C57BL/6 × 129 × CBA background.
Figure 1. a & b. [GR expression in control and FBRKO mice].
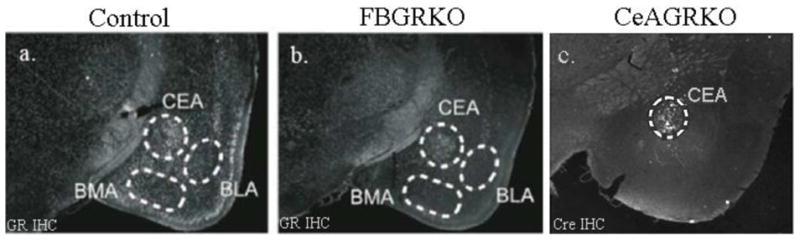
GR immuno-reactivity (white nuclei) is detected throughout neuronal populations in control mice (a.), but is markedly reduced or absent in the cortex, basolateral nucleus of amygdala (BLA) and basomedial amygdala (BMA) in FBGRKO mice. Note that GR expression is intact in the CeA and thalamus of the FBGRKO mice. c. [Cre-recombinase and GR staining in CeAGRKO mice]. Immunohistochemistry showing Cre immunoreactivity 2 weeks after LV-Cre infection
CeAGRKO
In order to establish the specificity of amygdalar GR disruption, we developed a lentivirus expressing Cre recombinase system delivered by stereotaxic injection into the brains of mice with a floxed GR allele (Fig. 1c) (Kolber et al., 2008a). Mice homozygous for the GRloxP allele were inbred on a C57BL/6 background. Three to four-month-old male mice were stereotaxically injected with either lentivirus (LV)-GFP or LV-Cre-recombinase. Briefly, anesthetized mice were mounted in a standard stereotaxic frame, a small hole was drilled on each side of the midline over the CeA and 4 × 105 infectious viral particles were injected into each CeA. In floxed GR mice injected with LV-Cre, we found disruption of GR in ~ 65% of CeA GR positive neurons with no changes in the BLA or other areas.
In the following sections, we will discuss the role of GR within specific brain nuclei in modulating fear conditioning, anxiety, and despair by evaluating behavioral alterations within these two mouse models developed within our laboratory.
3. Fear conditioning
Multiple lines of evidence have previously suggested that the amygdala is a critical component of fear-based learning and memory. However, key questions regarding the underlying molecular mechanisms and regional nuclear specificity still remained unanswered. Fear conditioning studies in our laboratory using CeAGRKO and control mice revealed that loss of CeA GR activity resulted in a deficit in contextual freezing and an attenuation of auditory-cued freezing (Kolber et al., 2008a). It is noteworthy to mention that no differences were found in corticosterone levels between CeAGRKO and control mice after training, indicating that the behavioral changes observed in CeAGRKO mice were due to reduced activation of GR in the CeA by normal levels of circulating glucocorticoids. Interestingly, we found no differences in fear conditioning (contextual or auditory-cued conditioning) when we compared the responses of FBGRKO and control mice in this task. The normal fear conditioning in FBGRKO mice suggested that potential viral-mediated off-target striatal or BLA deletion of GR was not sufficient to cause the conditioned fear deficits observed in CeAGRKO mice. Furthermore, we found increased corticotropin-releasing hormone (CRH) mRNA in the CeA of control mice after conditioned fear testing but not in CeAGRKO mice. Intracerebroventricular (ICV) infusion of CRH induced an increase in freezing behavior in both contextual fear and auditory cued behavior in CeAGRKO mice compared with CeAGRKO mice given control artificial cerebrospinal fluid injections. The results of these studies provide evidence to support a specific role for CeA GR in fear memory acquisition and consolidation and implicate CeA CRH as an important modulator of GR activity, and more generally, these data provide support to the growing body of evidence that the CeA plays a major role in fear conditioning (Kolber et al., 2008a, Pitts et al., 2009).
4. Anxiety-related behavior
Open-field testing was used to measure innate anxiety-related behaviors in both FBGRKO and CeAGRKO mice. No differences were observed in anxiety-like behaviors between FBGRKO and control mice under basal, nonstressed conditions (Boyle et al., 2006). Likewise, open-field testing revealed no basal differences between CeAGRKO and control mice (Kolber et al., 2008a).
Light/dark preference (LDP) is another commonly used measure of anxiety-like behaviors in rodents. Evaluation of LDP behaviors revealed a novel phenotype in FBGRKO mice. These mice entered the light compartment significantly earlier than controls, a pattern typically suggestive of decreased anxiety-like behavior. However, no basal differences were observed in the total amount of time spent in the light compartment between FBGRKO and control mice. As anticipated, restraint-stress, which would serve to increase anxiety-like behavior, produced a decrease in time spent in the light compartment in control mice, reflecting increased anxiety-like behavior. In FBGRKO mice, restraint stress had no effect on time spent in the light compartment. After treatment with the tricyclic antidepressant imipramine, control mice significantly increased the amount of time spent in the light compartment, suggesting that antidepressant treatment was effective in reducing anxiety in controls. Imipramine treatment in FBGRKO mice resulted in a modest decrease in the amount of time spent in the light chamber, suggesting that antidepressant treatment was not effective in reducing anxiety in FBGRKO mice.
Finally, the elevated-plus maze (EPM) was implemented as a final measure of anxiety-related behaviors in FBGRKO mice. FBGRKO mice spent significantly more time in the open arms and traveled a significantly greater distance in the open arms compared with control mice (Boyle et al., 2006), a behavior typically thought to reflect an anxiolytic-like phenotype. However, an alternative interpretation of EPM data from FBGRKO mice relates the increased time spent and distance traveled in the open arms of the EPM to increased agitation in response to the stress associated with the EPM task itself as opposed to reflecting a true decrease in anxiety-like behavior.
5. Locomotor behavior
Open-field testing was used to measure general locomotor activity in FBGRKO and CeAGRKO mice (Boyle et al., 2006; Kolber et al., 2008a). No differences were observed in locomotor activity under basal conditions between FBGRKO and control mice suggesting that, in general, FBGRKO mice are not hyperactive. However, during EPM testing, FBGRKO mice showed increased locomotor activity in both the open and closed arms. We suggest that the lack of difference in activity observed in the open field test may result from the open field representing a more natural environment that may not achieve a threshold stressful stimulus to distinguish genotypes. Differences in general locomotor activity were also measured in the LDP test between FBGRKO and control mice. In response to 30 minutes of restraint stress, FBGRKO mice demonstrated increased locomotor activity compared to control mice (Boyle et al., 2006). We suggest that the increased locomotor activity observed in FBGRKO mice may not reflect diminished anxiety-like behavior but rather may represent an exaggerated behavioral response in reaction to stressful conditions.
A sensory-motor battery and open-field testing were used to measure locomotor behavior in CeAGRKO mice (Kolber et al., 2008a). No differences in locomotor parameters were observed between CeAGRKO and control mice in any of the sensory-motor tests or open-field testing indicating that CeAGRKO mice do not display alterations in locomotor behavior under these low stress conditions.
6. Despair-like behavior
The forced swim and tail suspension tests are both standard measures of behavioral despair routinely used as a screen for rodent models of depression. Decreased activity in both tests is believed to represent increased despair-like behavior. The forced swim test revealed a significant decrease in activity in naïve FBGRKO mice compared to controls (Boyle et al., 2005). Likewise, the tail suspension test confirmed a reduction in activity demonstrating that forebrain GR activity mediates despair-like behaviors. Imipramine reversed this effect in both paradigms suggesting that the despair-like behavioral phenotype associated with loss of GR forebrain activity is reversed with antidepressant treatment (Boyle et al., 2005).
Anhedonia, or the loss of pleasure in enjoyable experiences, is commonly measured using the sucrose preference test in rodents. When presented with a choice between a bottle containing water and a bottle containing a sucrose solution, FBGRKO mice consumed significantly less of the sucrose solution compared to control mice, suggesting an increase in anhedonia in these mice (Boyle et al., 2005). Taken together, these data provide evidence that GR activity in the forebrain regulates the neural circuitry underlying despair-like behaviors. Current studies are underway to evaluate despair-like behavior in CeAGRKO mice to assess the role of CeA GRs in mediating despair-related behavior.
7. Conclusion
In this review, we summarize the behavioral phenotype of FBGRKO and CeAGRKO mice and discuss how studies using these mice have contributed to the understanding of the region-specific role of GR in the etiology of anxiety, despair and fear conditioning. Comparison of mice generated by Tronche and colleagues with our FBGRKO model offers important insights into delineation of the sub-regional role of GR. Mice with a nervous system specific knockout of GR (GRnes/cre) (Tronche et al., 1999) have deletions in a number of anatomically and functionally unique structures that disrupt GR signaling in areas that may have opposing effects on endocrine and behavioral output. These mice show hyperactivity of the HPA axis and decreased anxiety- and despair-related behaviors. The anxiolytic phenotype observed in these mice may appear to contradict the idea that decreased GR expression increases the activity of the HPA axis thereby increasing anxiety-related behavior. However, GR in different parts of the brain may differentially regulate the HPA axis and/or behavior. Maintenance of PVN GR activity in FBGRKO mice allowed us to evaluate the role of extrahypothalamic GR function in mediating stress-activated HPA axis activity and behavior. FBGRKO mice display hyperactivity of the HPA axis, similar to GRnes/cre mice, and increased depression-like behavior, in contrast to GRnes/cre mice. Taken together, these data define the involvement of forebrain GR in regulation of the HPA axis and highlight the importance of region-specific GR activity in behavior modulation.
Generation of FBGRKO mice have further contributed to our understanding of the role of GR by demonstrating that a primary defect in forebrain GR function leads to alterations in behavior and HPA axis regulation in response to stress. Additionally, we have shown that antidepressant treatment is ineffective at reversing the apparent anxiety-like phenotype of FBGRKO mice (Boyle et al., 2006), unlike despair-related behaviors, which are reversed after similar antidepressant treatment (Boyle et al., 2005). These findings suggest that although forebrain GR signaling is involved in mediating both anxiety- and despair-like behaviors, different pathways may modulate these responses. Generation of CeAGRKO mice has allowed for insights into the specific role of amygdalar GR activity which had previously been limited in part due to inadequately specific means for genetic manipulation of CeA function. Behavioral studies utilizing CeAGRKO mice have demonstrated that disruption of GR specifically within the CeA results in an attenuation of freezing during both contextual fear and auditory-cued testing associated with a decrease in CRH expression. Furthermore, the attenuation of both contextual and auditory-cued freezing is reversible with delivery of CRH (Kolber et al., 2008a). These findings provide evidence that the CeA is a site of memory acquisition and consolidation, and that at least part of this role can be attributed to GR.
In conclusion, these mouse strains provide a useful tool to further study the molecular alterations that underlie specific behavioral features such as despair and fear conditioning and provide important implications for the pathogenesis and therapy of psychiatric and stress-related disorders. A future challenge will be to use these mice to further delineate the neural circuitry involved in GR signaling and identify downstream modulators of GR activity.
Acknowledgments
This work was supported by National Institutes of Health grant NIH (MH079010) to L.J.M.
Abbreviations
- GR
glucocorticoid receptor
- CeA
central nucleus of amygdala
- HPA
hypothalamic-pituitary-adrenal
- GREs
glucocorticoid response elements
- BLA
basolateral nucleus of amygdala
- BMA
basomedial nucleus of amygdala
- CRH
corticotropin-releasing hormone
- ICV
intracerebroventricular
- LDP
light dark preference
- EPM
elevated-plus maze
- OF
open field
- CaMKIIα
calcium/calmodulin-dependent kinase II
- FBGRKO
forebrain-specific disruption of GR
- LV
lentivirus
- ACTH
adrenocorticotropic hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barden N, Stec IS, Montkowski A, Holsboer F, Reul JM. Endocrine profile and neuroendocrine challenge tests in transgenic mice expressing antisense RNA against the glucocorticoid receptor. Neuroendocrinology. 1997;66:212–220. doi: 10.1159/000127240. [DOI] [PubMed] [Google Scholar]
- Beato M, Herrlich P, Schutz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci U S A. 2005;102:473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;26:1971–1978. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Khor B, Vogt SK, Muglia LM, Fujiwara H, Haegele KE, Sleckman BP, Muglia LJ. T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat Med. 2003;9:1318–1322. doi: 10.1038/nm895. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci U S A. 2008a;105:12004–12009. doi: 10.1073/pnas.0803216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber BJ, Wieczorek L, Muglia LJ. Hypothalamic-pituitary-adrenal axis dysregulation and behavioral analysis of mouse mutants with altered glucocorticoid or mineralocorticoid receptor function. Stress. 2008b;11:321–338. doi: 10.1080/10253890701821081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montkowski A, Barden N, Wotjak C, Stec I, Ganster J, Meaney M, Engelmann M, Red R, Landgraf R, Holsboer F. Long -Term An tide pressant Treatment Reduces Behavioral Deficits in Transgenic Mice with Impaired Glucocorticoid Receptor Function. J Neuroendocrinol. 1995;7:841–845. doi: 10.1111/j.1365-2826.1995.tb00724.x. [DOI] [PubMed] [Google Scholar]
- Pariante CM. Depression, stress and the adrenal axis. J Neuroendocrinol. 2003;15:811–812. doi: 10.1046/j.1365-2826.2003.01058.x. [DOI] [PubMed] [Google Scholar]
- Pepin MC, Pothier F, Barden N. Impaired type II glucocorticoid-receptor function in mice bearing antisense RNA transgene. Nature. 1992;355:725–728. doi: 10.1038/355725a0. [DOI] [PubMed] [Google Scholar]
- Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J Neurosci. 2009;29:7379–7388. doi: 10.1523/JNEUROSCI.0740-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, Bilang-Bleuel A, Droste S, Linthorst AC, Holsboer F, Gesing A. New mode of hypothalamic-pituitary-adrenocortical axis regulation: significance for stress-related disorders. Z Rheumatol. 2000;59(Suppl 2):II/22–25. doi: 10.1007/s003930070013. [DOI] [PubMed] [Google Scholar]
- Steckler T, Holsboer F, Reul JM. Glucocorticoids and depression. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13:597–614. doi: 10.1053/beem.1999.0046. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Urani A, Gass P. Corticosteroid receptor transgenic mice: models for depression? Ann N Y Acad Sci. 2003;1007:379–393. doi: 10.1196/annals.1286.037. [DOI] [PubMed] [Google Scholar]


