Fig. 9.
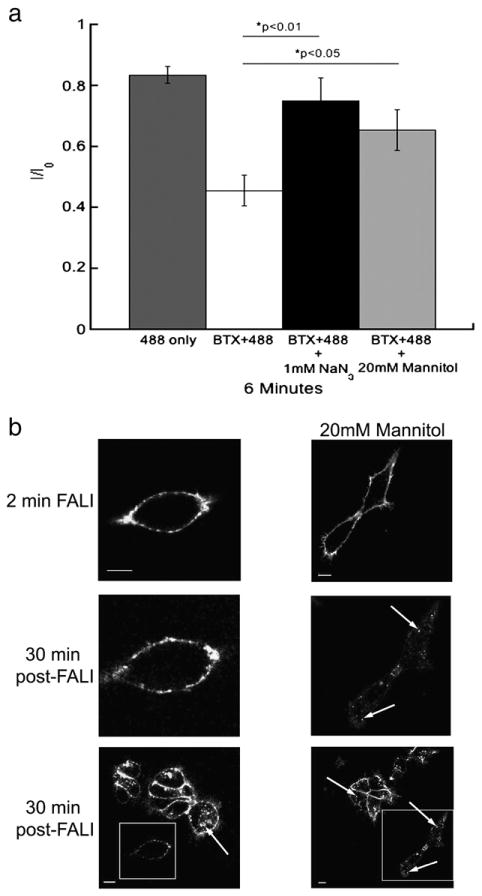
Fluorescence-induced inactivation of internalization and function of 5-HT3A/BBS receptors is reduced by reactive oxygen species scavengers. a) Average peak 5-HT-evoked current amplitudes recorded from HEK-293 cells stably expressing 5-HT3A/BBS receptors expressed as a proportion of the initial current (I0) after 6 min of ∼488 nm. Cells were exposed to 9.73 mW/cm2 of ∼488 nm light from a 100 W mercury arc lamp. Cells were either exposed to light alone (dark gray bars), or were labeled with BTX/488 and exposed to light in the absence of ROS scavengers (white bars). In the presence of 1 mM sodium azide (black bars) or 20 mM mannitol (light gray bars) an ANOVA analysis with a post-hoc Tukey test revealed that both 1 mM sodium azide (p<0.01) and 20 mM mannitol (p<0.05) reduced the fluorescence-induced inhibition of 5-HT3A/BBS receptor function. b) HEK-293 cells stably expressing 5-HT3A/BBS receptors and labeled with BTX/488 were exposed to 157.5 mW/cm2 of ∼488 nm light for 2 min (top row) in the absence (left column) or presence (right column) of 20 mM mannitol. After 30 min at 37 °C the same cell was imaged to monitor receptor internalization (middle row). A lower magnification image was also taken after 30 min at 37 °C to compare to surrounding cells that were not exposed to ∼488 nm light initially (bottom row). Boxes indicate cells exposed to light for 2 min and arrows indicate internal puncta. Scale bars=10 μm.
