Figure 2.
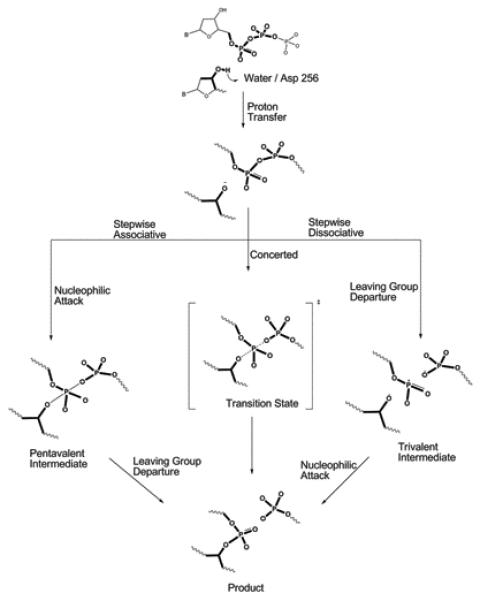
A schematic description of the chemical steps of the nucleotide incorporation reaction of Pol β. Deprotonation of the primer 3′-hydroxyl is followed by nucleophilic attack on the Pα of the incoming dNTP. Formation of O3′- Pα bond results in releasing a pyrophosphate group (leaving group). As shown in the Figure, after the initial proton transfer, the reaction may proceed either via a stepwise (associative or dissociative) or concerted mechanism. However, previous studies (ref.103) suggested that the hydrolysis of a phosphate diester is unlikely to proceed via a stepwise dissociative mechanism. In the stepwise associative model, a pentacovalent intermediate is formed between the bond-forming and breaking steps, resulting in two separate activation barriers. In the concerted model, reaction proceeds through a single transition state with partial bond forming and bond breaking character at the Pα.
