Figure 5.
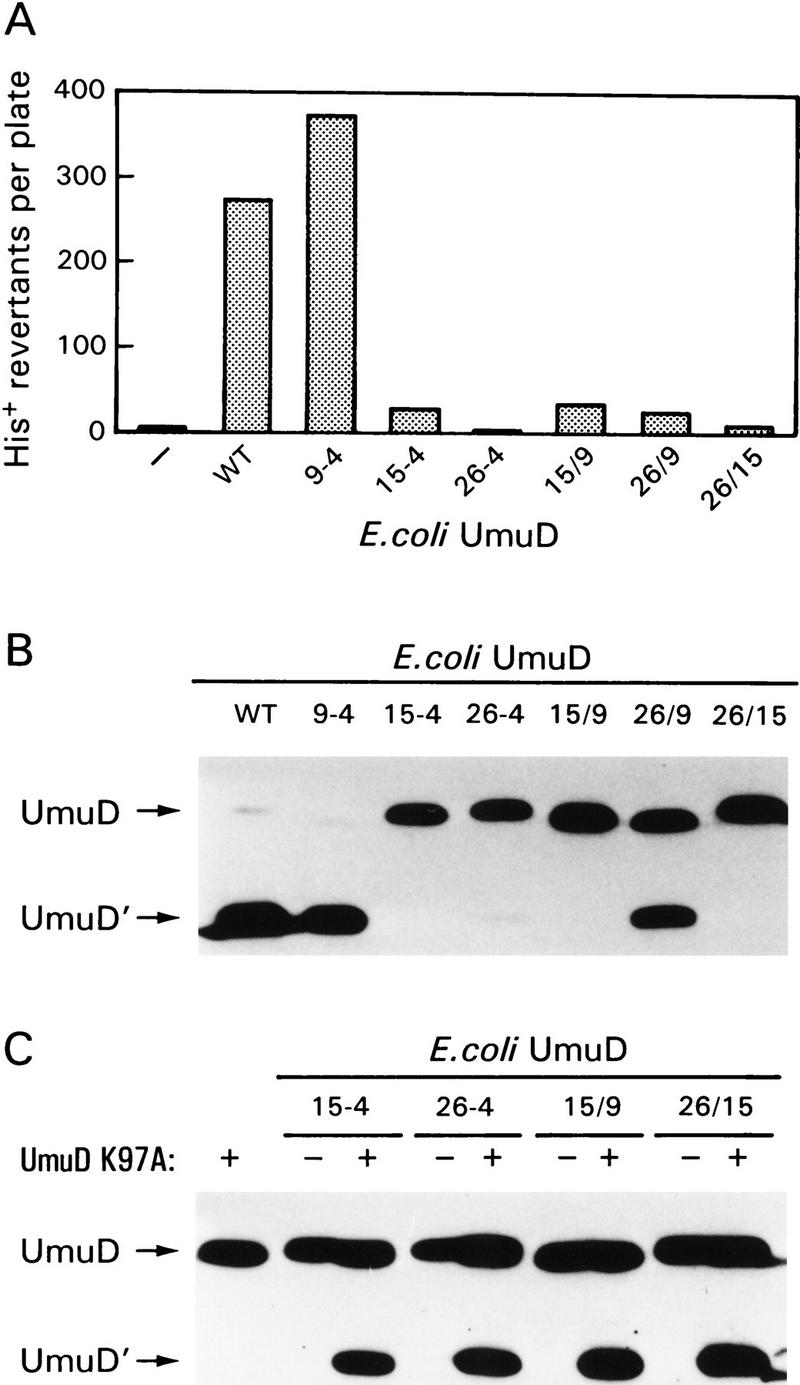
Functional activity of the UmuD alanine-stretch mutants. (A) The ability of the alanine-stretch mutants to function in SOS mutagenesis was assayed using strain RW126 harboring the low-copy-number UmuC-expressing plasmid pRW274 and a compatible plasmid expressing each of the individual UmuD alanine mutants. Strain RW126 carries an ocher mutation in hisG [hisG4(Oc)] that renders it auxotrophic for histidine. In the presence of functional Umu proteins it can, however, revert to His+. The number of His+ revertants per plate after exposure to MMS represents the mean number from a minimum of three cultures. (B) Western blot analysis of the RecA-mediated self cleavage reaction in vivo. The ability of the alanine-stretch mutants to undergo post-translational cleavage was assayed in the lexA51(Def) recA730 strain RW244. This strain constitutively expresses all LexA-regulated proteins (including the Umu proteins) and promotes constitutive cleavage of UmuD in the absence of exogenous DNA damage. The positions of UmuD and UmuD′ are indicated by arrows at left. (C) The ability of the UmuD alanine mutants to act as an enzyme in the intermolecular UmuD cleavage reaction. The UmuDK97A protein cannot undergo intramolecular cleavage because it carries a mutation at the active site of UmuD. It can, however, serve as a substrate in the intermolecular cleavage reaction if a UmuD enzyme is provided in trans (in our case, the various alanine-stretch mutants). Cleavage of the UmuDK97A protein was monitored in strain RW244 in the absence or presence of the individual UmuD alanine mutants and visualized by Western blot analysis as described in Materials and Methods. The positions of UmuD and UmuD′ are indicated by arrows at left.
