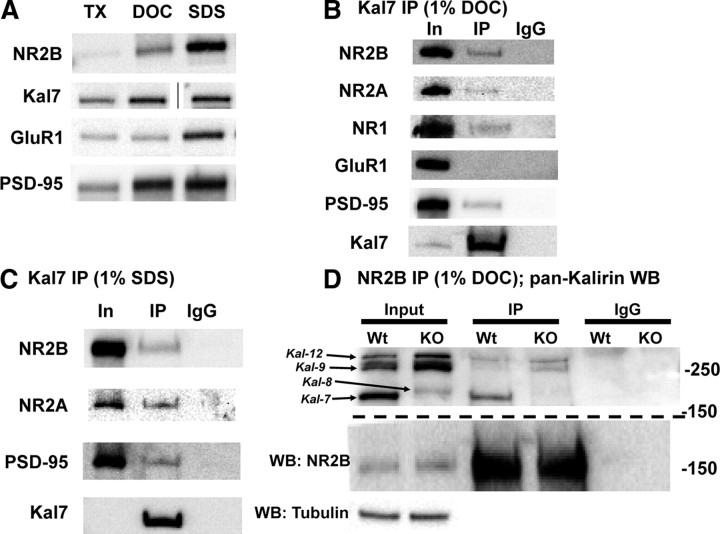
Figure 5.
Kal7 associates with NMDA receptor complexes in vivo. A, Solubilization of rat forebrain synaptosomal proteins with different detergents yielded different amounts of soluble NR2B, Kal7, GluR1, and PSD-95. Samples were loaded as equal percentages of the supernatant after detergent extraction; line in Kal7 sample indicates image is from a nonadjacent well of the same gel. B, In samples solubilized with DOC, immunoprecipitation (IP) with the Kal7 mAb coprecipitates a small fraction of the NR2B, NR2A, NR1, and PSD-95, but no detectable GluR1. Incubation of sample with preimmune IgG showed no binding. IP came from 50-fold more sample than Input (In); recovery of Kal7 is shown in the bottom panel. C, Solubilization of synaptosomes with 1% SDS revealed coprecipitation of NMDAR subunits and PSD-95 with Kal7; IgG controls again showed no binding. IP came from 100-fold more sample than Input; recovery of Kal7 is shown in the bottom panel. D, Synaptosomes from Wt and Kal7KO animals were solubilized with DOC, immunoprecipitated using an NR2B antibody, and blotted for all isoforms of Kalirin. Examination of the Input sample confirmed the absence of Kal7 and increase in Kal8/9/12 in Kal7KO animals, as reported (Ma et al., 2008b). A fraction of the Kal7 coimmunoprecipitated with NR2B in Wt extracts, as did small amounts of Kal9 and Kal12. In Kal7KO samples, coimmunoprecipitation of Kal9 and Kal12 was more apparent. Western blotting (WB) for tubulin confirmed equal loading in the input lanes, and IgG controls confirmed the absence of nonspecific binding; recovery of NR2B is shown in the middle panel. Similar results were observed in triplicate and quadruplicate (B, C) or duplicate (D) samples.