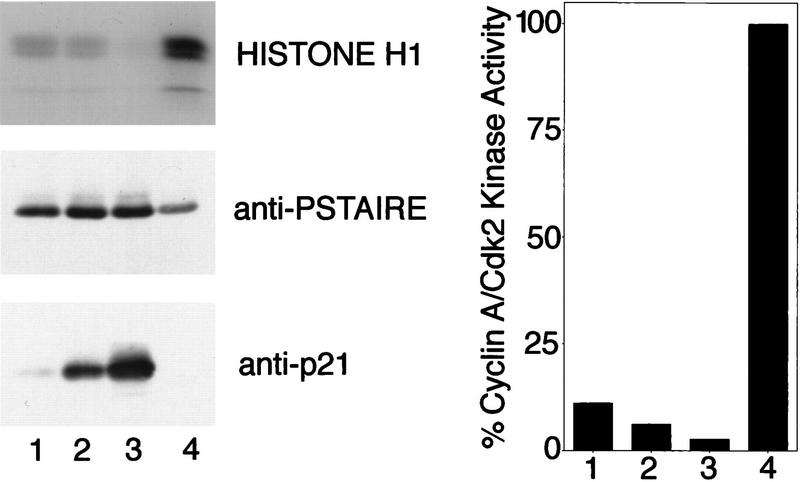
Figure 2.
Inhibition of cyclin A/Cdk2 by immobilized p21. (lanes 1–3) or anti-cyclin A (lane 4) antibodies were bound to protein A–Sepharose beads. These beads were incubated with varying amounts of purified p21: (lane 1) 0.05 μg; (lane 2) 0.2 μg; (lane 3) 0.2 μg; (lane 4) 0 μg. All beads were washed and incubated with ∼ 0.2 μg cyclin A/Cdk2. Nonbound kinase was removed from the beads and the sample of lane 3 was incubated subsequently with additional 0.4 μg of p21. The nonbound p21 was removed and the kinase activities associated with all samples were determined using [γ-32P]ATP and histone H1 as substrates. (Left) The amount of precipitated p21 protein (anti-p21) and Cdk2 (anti-PSTAIRE) was determined in Western blots using monoclonal antibodies. The level of 32P incorporation in the histone H1 bands (histone H1) was determined after SDS-PAGE by autoradiography of the dried gel. The amount of 32P incorporation in the histone H1 bands shown in this autoradiograph was measured using a PhosphorImager. The quantities of precipitated cyclin A and Cdk2 were determined by densitometric scanning of anti-PSTAIRE and anti-cyclin A Western blots and the histone H1 kinase activity of the samples is shown after normalization for the amount of precipitated kinase protein (right).