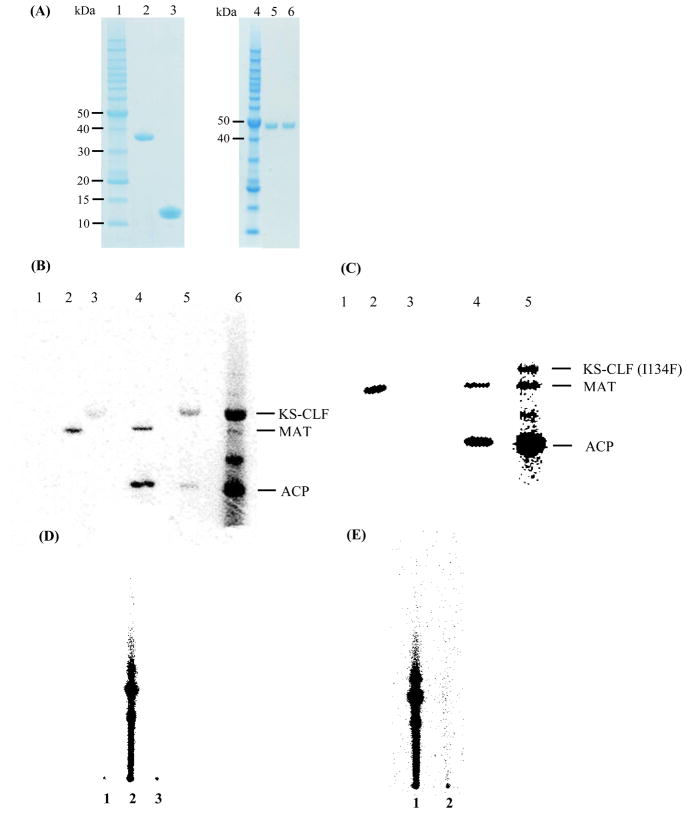
Figure 2.
(A) SDS-PAGE analysis of purified components of the fdm minimal PKS. Lane 1, protein molecular weight standard; lane 2, S. coelicolor MAT; lane 3, holo-ACP; lane 4, protein molecular weight standard; lane 5, KS-CLF I134F mutant; lane 6, wild-type KS-CLF. The two subunits have nearly identical molecular masses (44 kD and 42 kD, respectively), and therefore cannot be resolved via SDS-PAGE. (B) Activity of the minimal fdm PKS. PKS components were individually or collectively incubated with [14C]-malonyl-CoA, and quenched after 30 min: Lane 1, ACP; Lane 2, MAT; Lane 3, KS-CLF; Lane 4, ACP + MAT; Lane 5, ACP + KS-CLF; Lane 6, ACP + KS-CLF + MAT. (C) Activity of the fdm PKS harboring the I134F CLF mutation. Lane 1, ACP; Lane 2, MAT; Lane 3, KS-CLF; Lane 4, ACP + MAT; Lane 5, ACP + KS-CLF + MAT. The labeled band between the MAT and ACP in lanes 6 (panel A) and 5 (panel B) has not been characterized, but has precedence in the context of other Type II PKSs (Dreier and Khosla, 2000). (D) Activity of purified fdm minimal PKS: Lane 2, Minimal PKS in the presence of [14C]-malonyl-CoA (quenched at 1 h); Lane 1, Control sample pre-incubated with cerulenin for 20 min; Lane 3, Control sample lacking the KS-CLF. (E) Activity of the fdm PKS harboring the I134F CLF mutation: Lane 1, Minimal PKS in the presence of [14C]-malonyl-CoA (quenched at 1 h); Lane 2, the fdm PKS harboring the I134F CLF mutation in the presence of [14C]-malonyl-CoA (quenched at 1 h).