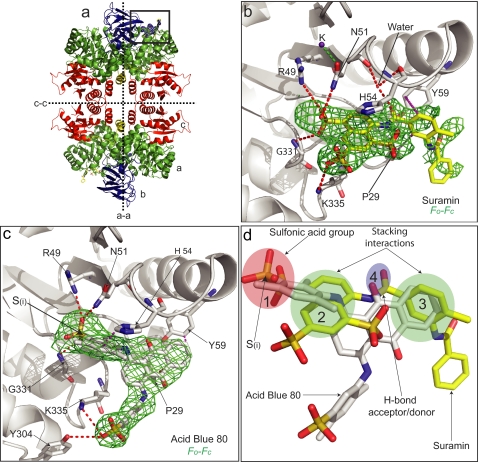
FIGURE 2.
Structures of LmPYK-suramin and LmPYK-AB80 complexes. a, the LmPYK-suramin tetramer in which the domains have been colored to aid identification (green, A domain; blue, B domain; red, C domain; yellow, N-domain). b, enlargement of the active site of the LmPYK-suramin structure. Suramin is shown by cyan sticks and corresponds primarily to one symmetrical half of the suramin molecule. The position of the suramin molecule is shown by an unbiased Fo − Fc electron density map contoured at 2 σ (orange). A K+ ion is shown as a purple sphere. c, AB80 bound in the active site of LmPYK. The AB80 molecule is shown with an unbiased Fo − Fc electron density map contoured at 2.5 σ (green). d, the LmPYK-AB80 monomer (colored gray) superposed onto the LmPYK-suramin structure (colored yellow); the orientation is identical to that of panel c. Overlapping binding characteristics of the sulfono group-containing molecules highlight key groups required for inhibitor binding; for clarity, only suramin and AB80 are shown. The four key binding characteristics observed in the LmPYK and TcPYK sulfonic acid structures are highlighted with colored ellipses as described previously in the legend for Fig. 1: site S1, pink ellipse, a sulfono group (S(i)) is observed bound in a near identical position in all complexes; site S2, green ellipse, a stacking interaction with His-54 is conserved in all structures; site S3, green ellipse, a second stacking interaction with Tyr-59 and Pro-29 is observed for suramin, Ponceau S, and AB80; site S4, blue ellipse, a hydrogen bound acceptor/donor is also commonly observed. Hydrogen bonds are shown as dashed red lines, and stacking interactions are shown as dashed purple lines.