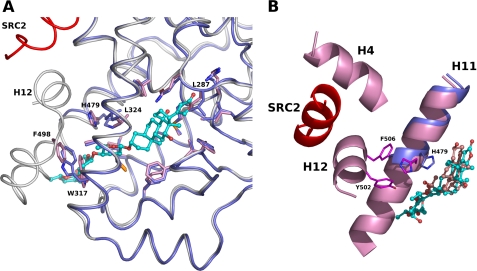
FIGURE 4.
Superposition of the RORγt LBDs in complex with digoxin and with the agonist. A, digoxin induces a conformational change of Trp-317. The LBD (dark blue) with digoxin and the LBD (white) with the agonist hydroxycholesterol are depicted as a cartoon model. The coactivator SRC2 peptide is colored in red. Residues of RORγt involved in the binding of digoxin are shown as a stick model, and the corresponding residues in the agonist-bound LBD are also depicted. B, digoxin disrupts cation-π interaction observed in the agonist-bound RORγt LBD. Cartoon representation of the helix H11 (dark blue) of the digoxin-bound RORγt LBD and helices H4, H11, and H12 (pink) of the hydroxycholesterol-bound RORγt LBD are shown. The SRC2 peptide is colored in red. Residues His-479, Tyr-502, and Phe-506 involved in the cation-π interaction in the agonist-bound RORγt LBD (carbon, magenta) are shown as a stick model. The side chain atoms of His-479 (carbon, dark blue) are moved and make a hydrogen bond with digoxin. Digoxin (carbon, cyan) and hydroxycholesterol (carbon, pink) are shown as a ball-and-stick model.