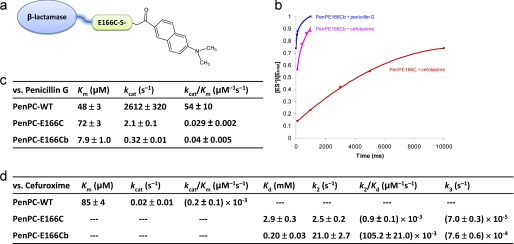
FIGURE 1.
The kinetic profile of BADAN-labeled β-lactamases. a, scheme for BADAN labeling at residue 166 of a β-lactamase enzyme. b, time course of ES* formation as monitored by ESI-MS when PenP-E166Cb was mixed with cefotaxime or penicillin G at a concentration of 2.5 μm (enzyme) versus 5 μm (substrate). Such spectra obtained at different substrate concentrations were used to calculate the kinetic parameters of the labeled enzyme following the procedure as previously reported (4). The solid squares represent the [ES*]/[Etotal] values of the PenP-E166C-antibiotic and PenP-E166Cb-antibiotic complexes respectively as determined by ESI-MS. Buffer: 20 mm ammonium acetate (pH 7.0). Curve fitting of the time profile was performed using single-binding site. c and d, kinetic parameters of the wild-type PenPC β-lactamase, its PenPC-E166C mutant, and the BADAN-labeled PenPC-E166Cb mutant for the hydrolysis of penicillin G (c) and cefuroxime (d). The kinetic parameters of the wild-type PenPC for the hydrolysis of cefuroxime were quoted from our previously published results (4).